Determining the use of antibodies
A new research project is necessary to understand some of the basic properties of the protein of interest, especially if it is known that the structure of the protein will be of great help in selecting areas where antibodies are easily accessible and recognized. However, without such precise structural information, understanding the use of research can affect the strategy of peptide design. For example, if the focus of the study is on different regions of the protein, such as the C-terminus or the N-terminus, or proteins in a particular state, such as phosphorylation, then the peptides designed according to the desired sequence and the corresponding antibodies produced are There should be no difficulty in application, however, the conformation of the protein will affect the interaction between the antibody and its recognition region. A problem that may exist in this case is that if the recognition region is hidden inside the protein in the folded protein and the antibody will not be able to contact the region, no interaction can occur.
Principle of selection of identification areas
Generally speaking, the most ideal antigen recognition region should be hydrophilic, located on the surface of the protein and structurally deformable. Because in most natural environments, hydrophilic regions tend to concentrate on the surface of the protein, and hydrophobic regions are often encapsulated inside the protein. Similarly, antibodies can only interact with recognition regions found on the surface of the protein, and when these are recognized When the region has sufficient structural deformability and is transferred to a position where the antibody can be contacted, it will have a high affinity with the antibody.
Continuous and discontinuous identification areas
A continuous region refers to an identification region composed of a continuous sequence of amino acids. Most antibodies are directed against a continuous recognition region, and the ability of antibodies to bind to such regions with high affinity indicates that the sequence is not inside the protein. A discontinuous recognition region is a segment of a polypeptide that represents a certain fold, or an identification region of an antibody that links two separate polypeptides together. In some cases, antibodies directed against such discrete recognition regions can also be produced, except that the antigenic polypeptide used for immunization must have a secondary structure similar to the discontinuous recognition region, and the length of the sequence needs to meet the relevant requirements.
Basic advice
In order to avoid the risk of the recognition region being hidden inside the protein, we usually recommend selecting the corresponding antibody at both ends of the N and C proteins. Because in intact proteins, both ends of N and C are usually exposed to the surface of the protein. However, it is important to note that the C-terminal hydrophobicity of membrane proteins is too strong to be suitable as an antigen.
Length of sequence
Generally, we suggest that the sequence length of the antigenic polypeptide is between 8-20 amino acid residues. If it is too short, there is a risk that the polypeptide specificity is not strong and the binding ability between the produced antibody and the native protein is not strong enough; however, If the sequence length exceeds 20, it is possible to introduce a secondary structure, and the resulting antibody loses the possibility of specificity, and the longer the peptide chain, the more difficult the synthesis is, and it is difficult to obtain a high-purity product.
Selection of carrier protein crosslinks
The carrier protein is added to the end remote from the antibody recognition region, and Cys is the preferred method for crosslinking at the N or C end in the absence of Cys in the sequence.
Ready-to-eat Sweet Corn Kernels
In one sunny morning, a sweet corn or glutinous corn is a good choice for breakfast to kick-start the day. Corn has a richer taste than other staple food. Glutinous corn has a sweet and waxy taste. It's also a filling food to fill up for the day, supplement the nutrition that the human body needs.
Fruit corn. The outer leaves are pale green, and the inner grains are white or yellow.The sweetness is higher than sweet corn, but the starch content is low, rich in vitamin E and cellulose, can fight aging and help digestion.Ideal for raw food, mixed with vegetables into salad;It can also be steamed, but don't cook it for too long, about five minutes.


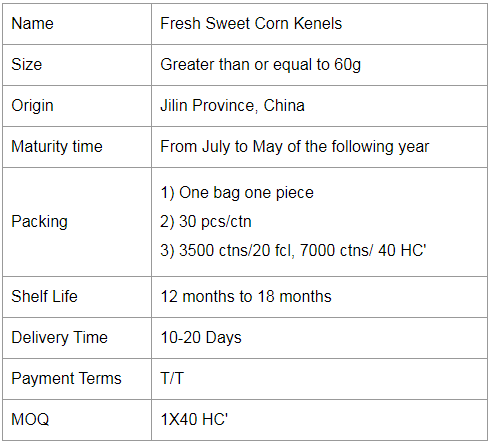
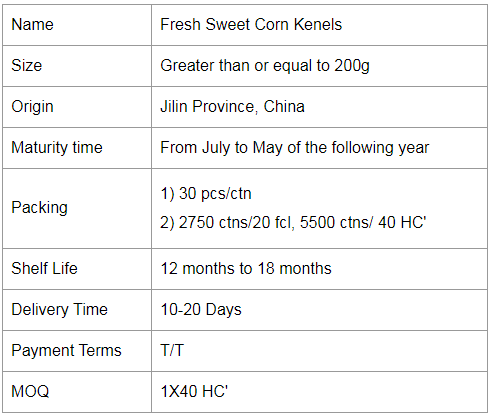
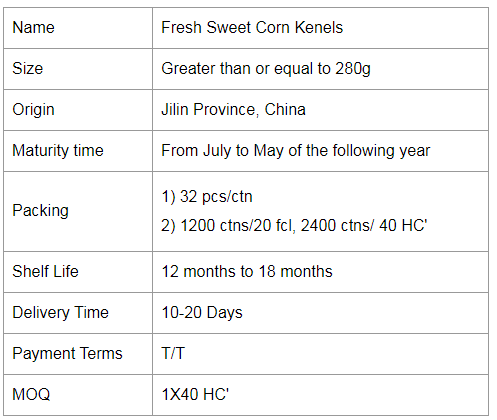
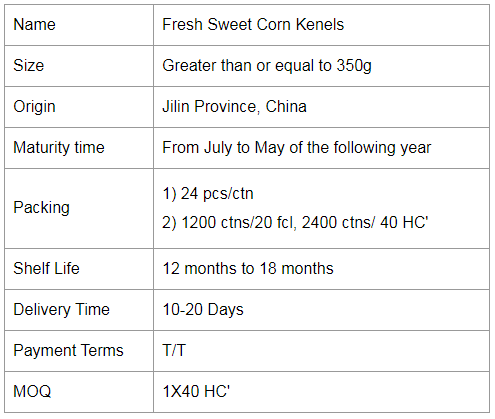
If you would like to work with us, please send us a message on the website or contact us by email.
Fresh Corn Kernels,Corn Whole Kernel,Boiled Corn Kernels,Baby Corn Kernels
Jilin Province Argricultural Sister-in-law Food Co., Ltd. , https://www.nscorn.com