Discussion on the development of Me-too drugs through case analysis
Discussion on the development of Me-too drugs through case analysis
August 6, 2018 Source: Author drugs crossing: Liubiggeneral
Window._bd_share_config={ "common":{ "bdSnsKey":{ },"bdText":"","bdMini":"2","bdMiniList":false,"bdPic":"","bdStyle":" 0","bdSize":"16"},"share":{ }};with(document)0[(getElementsByTagName('head')[0]||body).appendChild(createElement('script')) .src='http://bdimg.share.baidu.com/static/api/js/share.js?v=89860593.js?cdnversion='+~(-new Date()/36e5)];The process by which some pharmaceutical companies have increased the rate at which new drugs are developed through various innovative technologies has become increasingly difficult. Even if the company invests huge sums of money in technology updates, its sales are still far behind the rapid increase in the cost of research and development of new drugs. With the emergence of the above problems and competition in the global pharmaceutical prices, the challenges of the genetic era. Pharmaceutical companies have begun to pay attention to the application of Me-too strategy in the research and development of new drugs. Experimental facts show that the discovery of new drugs by Me-too strategy can greatly increase the rate of development of new drugs. More and more companies are beginning to apply Me-too strategies to modify and modify existing drug molecules. Successful instance.
Me-too study of H2 receptor antagonists
In the past, in order to find drugs to inhibit gastric acid secretion and treat digestive ulcers, American Shike Company spent a lot of effort based on the theory of H2 receptor antagonists. It took more than ten years to successfully develop cimetidine, which has a chemical structure consisting of a five-membered ring of imidazole, a tetraatomic chain containing a thioether, and a terminally substituted èƒ3 moiety, making it the first highly active H2 receptor. The body antagonist drug was first marketed in the UK in 1976. It soon became the drug of choice for the treatment of ulcer disease, and in 1979 it was licensed in more than l100 countries. The advent of cimetidine has opened up new areas for the treatment of ulcer disease. After the scientists of Glaxo knows about the possibility of developing a new drug for H2 receptor antagonists, Zeng Yiding's imidazole ring is the necessary structure for the recognition of this class of drugs and H2 receptors. The early transformation work focused on the side chain. The change, but did not get better drugs than cimetidine. Later, the imidazole ring of histamine was replaced by a furan ring, and Ranitidine was introduced in 1981. In 1983, ranitidine became the second listed H2 receptor antagonist, which is 5-8 times more effective than cimetidine. It has high curative effect on the stomach and duodenum, and has quick-acting and long-acting characteristics. Its side effects are smaller than that of cimetidine, and it has no anti-androgenic side effects, and its interaction with other drugs is also small. As a result, it has become the world's best-selling drug, with an annual sales volume of US$3 billion, ranking first in the world's best-selling drugs for several consecutive years.
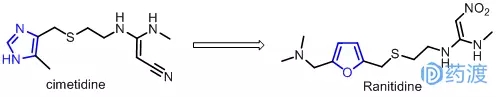
Me-too study of angiotensin converting enzyme inhibitor
The development of angiotensin-converting enzyme (ACE) inhibitors, a large amount of early work was done at the university. At that time, it did not cause people's commercial pursuits. It was not until the late 1970s that Captopril was successfully developed. It was first listed in the United States in 1981. Angiotensin-converting enzyme has a zinc ion, and sulfhydryl (-SH) is a site that binds to zinc ions, but captopril also has a scent of garlic with a sulfhydryl group. Merck then introduced Enalapril in 1984. This variety has been removed from the thiol group in the molecule. It contains ester groups as prodrugs, which can improve absorption, enter the center, and hydrolyze in the body. It is affinity with the active center and enhances the strength of binding to the enzyme. The therapeutic effect is 10 times stronger than that of captopril, and the toxicity is small and the effect is long-lasting. In 1995, enalapril achieved global sales of $2.5 billion. In the 1990s, BM Sqllibb introduced Fosinopril, a novel angiotensin-converting enzyme inhibitor containing phosphorus, which is a prodrug and has a weak direct inhibitory effect on ACE, but after oral administration. Slow and not fully absorbed. And quickly converted to a more active diacid metabolite Fosinoprilat (Fosinoprilat), long-lasting effect. Fosinopril inhibits ACE activity through the combination of its hypophosphorus group and zinc ion in the active site of ACE. Fosinopril has no significant effect on renal function and is a representative of the third generation of ACE inhibitors.
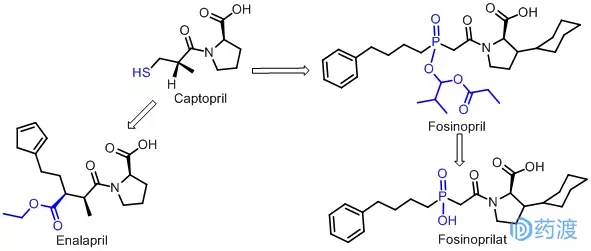
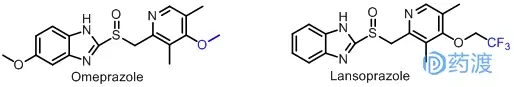
Me-too study of proton pump inhibitors
Omeprazole, the first proton pump inhibitor developed by Astm, was launched in 1988 and has a strong and long-lasting effect on stomach acid. Lansoprazole is a derivative of omeprazole, which has the same anti-acid secretion effect as omeprazole, but its stability and oral availability are significantly improved compared with omeprazole. Structurally, lansoprazole and omeprazole are almost identical except for a fluorine-substituted alkyl group.
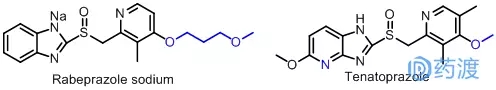
In recent years, many manufacturers at home and abroad have produced a large number of new drugs based on the structure of omeprazole and lansoprazole and the research method of "Me-too medicine". For example, in 1998, the company was listed in Japan, Rabeprazole sodium, which was introduced to Europe in 1999. In the same year, it was approved by the US FDA for listing in the United States, bringing a new treatment option to foreign countries in a short period of time. Physician's approval for many patients with peptic ulcer and gastroesophageal reflux disease, structural analogy can be seen as a structural analog of omeprazole, its structure is very similar to lansoprazole, only a slight change in the side chain The original trifluoroethoxy group was changed to methoxypropoxy group, and the other parts were basically the same, and it was also a kind of "Me-too medicine". Tenatoprazole, marketed in Japan in 2004, is a novel gastric H+/K+-ATPase inhibitor developed by Japan's Tokyo Tanabe, Mitsubishi Corporation of Japan and Hokuriku Pharmaceutical Co., Ltd. (ie proton pump suppression) Agent). The drug significantly inhibits the secretion of gastric acid and also inhibits H. pylori. The effect is 7 times stronger than omeprazole, and the stability is also significantly improved compared with omeprazole. In terms of its structure, the benzene ring in the omeprazole structure is replaced with a pyridine ring to produce another new drug.
Me-too study of dihydropyridine calcium antagonists
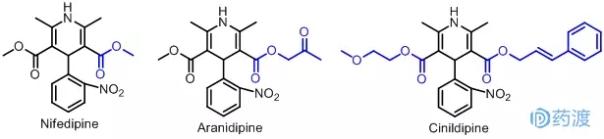
Dihydropyridine calcium antagonists are a class of highly specific and potent drugs with strong vasodilator effects, which are suitable for coronary spasm, hypertension, and myocardial infarction. Nifedipine was first used. A series of "dipine" compounds were synthesized based on structural modification of structure-activity relationship: Aranidipine was introduced by Taiho Corporation of Japan in 1996. A new drug of dihydropyridine. It is modified on the basis of nifedipine to replace the methyl hydrogen on the ester group at the 3-position of the dihydropyridine ring with an acetyl group. The drug is capable of stereoscopically inhibiting calcium channels, and is slower to bind and dissociate from the receptor, and has a longer-lasting effect on blood pressure lowering than nifedipine. Cinildipine is a long-acting dihydropyridine calcium antagonist marketed by Japan Fuji Co., Ltd. in 1996. It is obtained by replacing the ester group at the 3,5 position of nifedipine. The double bond is a hindrance to calcium channels and a long-acting antihypertensive drug. The latter two drugs are new drugs based on the structure of nifedipine, which are classic representatives of Me-too drugs.
Me-too study of hydroxymethylglutaryl coenzyme A reductase inhibitor
In the early 1970s, several microbiologists such as Endo of the Beili Institute of Medicine in Japan discovered an accident that inhibited the activity of hydroxymethylglutaryl coenzyme A (HMG-CoA) reductase. Significantly lowers cholesterol in the plasma. Japanese scientists named the newly discovered substance "Compactin", which is mevastatin. The discovery of mevastatin opens a new era in the search for and development of HMG-CoA reductase inhibitors to regulate blood lipids. However, due to the fact that the industrial technology at that time was not completely cleared, and Japanese pharmaceutical companies did not invest heavily in mevastatin and other derivative products, they continued to develop intensively, eventually failing to form production capacity and losing statins to regulate blood lipids. The development advantage of medicine.
However, Japanese scholars' research on statin lipid drugs has aroused strong interest from Western medical colleagues. The old pharmaceutical industry powers such as the United States, Germany, Britain, France and Switzerland have invested a lot of manpower and financial resources in the research and development of statins, and have achieved fruitful results. In less than 20 years, Western countries have developed more than 10 statins to regulate blood lipids, including mevastatin. Developed by Merck, lovastatin (Lovastatin) was first marketed in the United States in 1987 as the first statin to be marketed. It is an inactive prodrug that needs to hydrolyze the ester ring into an open-chain hydroxy acid in vivo. The derivative has anti-enzymatic activity, which can competitively inhibit HMG-CoA reductase, reduce cholesterol synthesis and apolipoprotein concentration; increase the activity of low-density lipoprotein receptor; and also reduce triglyceride in plasma by a small amount. And the concentration of cholesterol in very low density lipoprotein, increase the cholesterol level in serum high density; with the combination of ester-lowering drugs have a good synergy. Simvastatin was successfully developed after lovastatin, which is a methylated derivative of lovastatin, which is twice as active as lovastatin. Merck was applied in Europe in 1981 and in the US in 1984. patent.
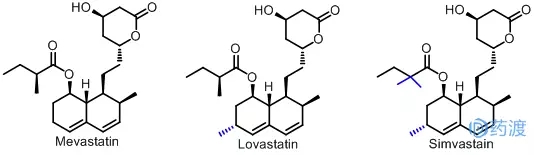
Subsequently, a series of Me-too drugs were produced, such as pravastatin developed by Japan Sankyo Company, fluvastatin developed by Swiss Mountain Taoist Company, rivastatin developed by Bayer Company of Germany, and Warner-Lambert Company of USA (now Into Pfizer) to develop atorvastatin and the like.
Me-too study of nitrogen mustards in bioalkylating agents
The discovery of nitrogen mustards stems from mustard gas. Mustard gas is an alkylating agent poison that was used as a poison gas during World War I. Later, it was found that mustard gas has a therapeutic effect on lymphoma. Because of its toxicity, it is impossible to use medicinal products. On this basis, nitrogen mustard anti-tumor drugs have been developed, so that the replacement of S atoms by N atoms has become the focus of the transformation of nitrogen mustard drugs.

From the structure of nitrogen mustard, R is a carrier moiety, which can improve the absorption, distribution, and other pharmacokinetic properties of such drugs in vivo, and other parts belong to the functional group of anti-tumor activity in the alkylated moiety. When the carrier moiety is an aliphatic hydrocarbon group, it becomes a fatty nitrogen mustard, and when it is substituted by an aromatic ring, it is an aromatic nitrogen mustard.
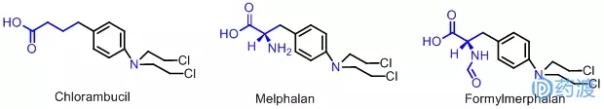
Studies on the structure-activity relationship of aromatic nitrogen mustards showed that the best effect was when the number of atoms between the carbon and benzene rings was 3, namely, chlorambucil (Chlorambucil), which was used to treat chronic lymphocytic leukemia. It has good curative effect on lymphosarcoma, Hodgkin's disease and ovarian cancer. It is clinically used with its sodium salt, which has good water solubility and is absorbed by the gastrointestinal tract. It is rapidly converted into free chlorambucil in the body.
Another successful example is the substitution of a side chain of an aromatic acid to introduce a naturally occurring amino acid in order to increase the concentration and affinity of the drug at the tumor site and to improve the efficacy of the drug. For example, Melphalan (lysin), which is based on phenylalanine, has a good effect on malignant tumors such as ovarian cancer, breast cancer, lymphosarcoma and multiple bone marrow.
Chinese scholars formformylation of NH2 on the basis of melphalan to obtain Formylmerphalan. Compared with sarcoma, the therapeutic index is high and the toxicity is low.
In the reality that China's patent system is increasingly perfect, the investment in new drug research and development funds is relatively insufficient, and the research level is relatively backward. The use of Me-too strategy to discover new drugs reduces technical difficulty, risk and R&D costs compared to new drug innovations, which can avoid patent infringement and accelerate the research of chemical synthetic drugs in China. To provide necessary technical accumulation and fund accumulation for new drug research and development work, and promote the development of China's pharmaceutical industry.
Reference materials:
You Qidong. Medicinal Chemistry
Wang Shuyue, Wang Hongliang. Talking about the status of Me-too medicine in the research of new drugs
Liu Ruiwu. Tailored Me-too New Drug
Disposable Piercing Guide - WPTC15
Laparoscopic instruments are medical devices used in minimally invasive procedures. These instruments are designed to be inserted through small incisions in the body, allowing surgeons to operate with less pain, scarring, and recovery time than traditional open surgery.
Advantages of Laparoscopic Instruments:
1. Smaller incisions: Laparoscopic instruments allow the use of smaller incisions, reducing scarring, pain and recovery time.
2. Improved visualization: Laparoscopic instruments provide a better view of the surgical site, allowing for more precise and accurate surgical procedures.
3. Reduced risk of infection: Compared with traditional open surgery, laparoscopic surgery usually has a lower risk of infection.
4. Reduced blood loss: Laparoscopic instruments are designed to minimize tissue trauma, thereby reducing blood loss during surgery.
5. Faster recovery: Compared with traditional open surgery, laparoscopic surgery usually has a faster recovery time, allowing patients to return to normal activities sooner.
Disposable Use Puncture Guider,All Laparoscopic Instruments,Keyhole Surgery Instruments,Surgical Laparoscopic Machine
Changzhou Weipu Medical Devices Co., Ltd. , https://www.cnweipumedical.com