Release date: 2015-08-06

The Ebola virus broke out in West Africa in December 2013, triggering the largest epidemic in Ebola's history, and there were no safe and effective vaccines or drugs. Just 20 months later, on July 31, the Lancet magazine published preliminary results in Guinea, and the newly developed vaccine can provide almost 100% comprehensive protection against infection. Focusing on the initial success of the trial, Nature 's comprehensive analysis of the impact of the new vaccine on the outbreak that has caused more than 11,000 deaths and how to conduct future clinical trials in the outbreak.
How did the vaccine come from?
The vaccine, called rVSV-ZEBOV, contains a livestock virus that is genetically engineered to be Ebola. It was developed by the Public Health Agency of Canada and licensed to Merck Pharmaceuticals and tested by funders, scientists, businesses, organizations and governments under international cooperation, including the World Health Organization (WHO). In Guinea, scientists use a “loop†arrangement (such as members of the same family) to vaccinate infected contacts. The vaccinated persons were divided into two groups: one group was vaccinated immediately after exposure, and the other group was vaccinated three weeks after exposure to the infected person.
What did the experiment find?
In 2014, even in vaccinated infected contacts, there was no case of Ebola infection after a 10-day window period (the window period gave the vaccinator enough time for an autoimmune response). In contrast, 16 of the 2,380 delayed vaccinated contacts were infected during this period. Therefore, the vaccine is believed to provide 100% protection against the virus in this trial.
100% protection sounds incredible
Marie-Paule Kieny, assistant director-general of the WHO Department of Health Systems and Innovation, said the study was quite small, so the true protection rate may be slightly lower than 100%. Another independent committee reviewed the trial and considered the preliminary results to be very convincing. In the new trial on July 26, the original control group was cancelled and all contacts were now given even if vaccinated. This will generate more useful data for evaluating the level of protection of the vaccine. However, the current results are enough to make everyone excited. Jesse Goodman, a former US Food and Drug Administration official at Georgetown University, commented: "This report is quite compelling. Even if there are some problems in the research, the vaccine seems likely to be effective."
What is the vaccine validity period?
The answer to this question is still unknown. The purpose of the trial was to test whether ring vaccination can contain disease outbreaks, and the protection known for several weeks is sufficient to do so. Adrian Hill, director of the Jenner Institute at the University of Oxford, UK, participated in other types of Ebola vaccine tests. He commented: "This is good news for the resolution of the disease outbreak, but it remains to be seen whether this protection will continue. The trial did not tell us the exact time of effective protection of the vaccine. "The vaccine that provides long-term, even life-long immune protection is what we really need. Other vaccine trials, including those involving Adrian Hill, are effective vaccines for testing long-term protection. But the number of Ebola cases that are falling means that trials may be difficult to provide clear results.
Can the rVSV-ZEBOV vaccine end Ebola's popularity in West Africa?
The vaccine will continue to be used in Guinea as part of a clinical trial. Many researchers hope to use it in Liberia and Sierra Leone (although the number of infections in the area has fallen sharply, but there is a risk of sustained attacks and spread to neighboring countries) to end the epidemic. However, some regulatory barriers need to be cleared first. WHO spokesperson Gregory Hartl said deployment arrangements in these countries may be part of an expanded clinical trial or an emergency mandate through regulatory authorities. The authorities are also considering whether the available data is sufficient to permit the use of the vaccine outside the clinical trial environment. This process can take several weeks to several months.
Is it unusual to experiment during a disease outbreak?
Yes. Clinical trials that require regulatory approval usually take several years (based on the gold standard for randomized controlled trials). This means that the outbreak is often nearing the end of the trial. Clinical trials are usually conducted in well-equipped research hospitals, and quality testing of new vaccines is generally considered impossible in the event of a fatal outbreak. And the urgency of Ebola's attack has changed all these rules. The WHO has worked hard to speed up the effective treatment and vaccine testing of animal experiments, reduce red tape, and propose relevant trial designs that can quickly provide data that at least helps with epidemic control. The rVSV-ZEBOV test is one of several related experimental designs.
Does this "fast track" approach apply to other diseases?
Adrian Hill believes that vaccines can be rapidly developed in the face of many other epidemiological threats. He suggested that research on specific pathogen vaccines be accelerated and that clinical trials to test vaccine safety can be completed in a timely manner. Those approved vaccines will be stored and used for efficacy testing once an outbreak occurs. Such pathogens that are a priority for health threats include Marburg virus (the same family of Ebola virus), Middle East Respiratory Syndrome (MERS) virus, Lassa fever and Chikungunya. ).
What lessons have you learned from the success of rVSV-ZEBOV?
Its success provides a model for dealing with future outbreaks. Adrian Hill said: "This means that accelerated development of vaccines is feasible." WHO Director-General Margaret Chan said on July 31 that they are developing a "blueprint" to accelerate the development of relevant measures to eliminate potential epidemics. The plan is designed to shorten the identification of outbreaks to relevant effective measures to less than four months, and to advance the approval of the trial design and regulatory authorities.
Source: Bio Valley
We have a variety of designs for Intercom System With Tuya, different sizes, different keys, etc., you can refer to our pictures,
We have been focusing on independent research and development of intercom system with tuya
since 2011. We have professional reach and devolve team to serve you, and we can provide you with professional products and services, If you need more information about our factory or intercom system, please do not hesitate to contact us, we are very honored to share catalog with you.
Smart doorphone with tuya is an intercom system that can be connected to a mobile phone. The system adopts a unified TCP/IP networking architecture to connect all-digital video intercom, security equipment and elevator linkage system. At the same time, it can be connected to the central management machine. The property community can better manage tenants, improve user experience and property services, etc. They use POE switches to connect multiple indoor units and video door phones, and POE switches supply power to the indoor units. The wiring is very simple and convenient, and the overall cost is low.
Smart doorphone with tuya improves user experience, because users can receive call information, and can monitor Video Door Phone at any time through tuya application, even if users are in an office, picnic or travel, they can immediately receive call information from visitors , and talk to them, you can also monitor visitors. If it is the user's friend, it can also remotely unlock the visitor on the mobile phone, which is very convenient.
The video doorphone in the picture is the most popular model from our company - MK-AZZJ4C, we have adopted the embedded design, which is very classic and beautiful. It is also an Android system, which can support simultaneous connection of multiple indoor units and mobile phones to realize remote monitoring, calling and unlocking on the mobile phone. We have also added the function of face recognition and LED fill light, which can clearly monitor and unlock using face recognition even at night. Ideal for multi-apartment, hotel and community applications.
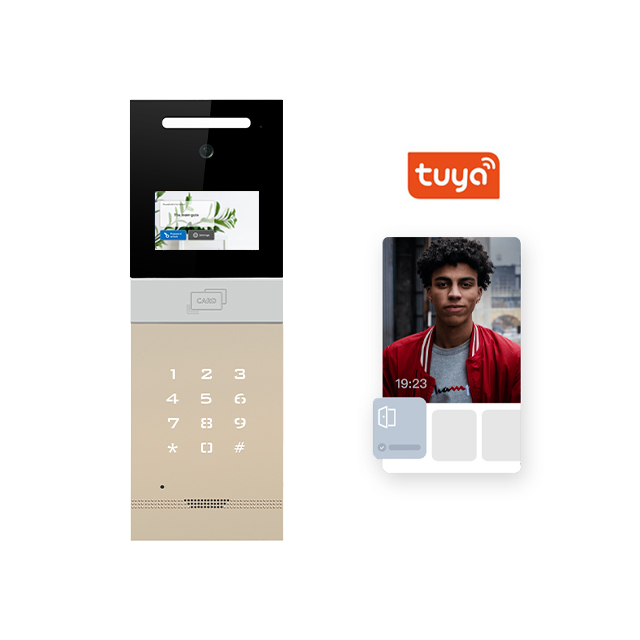
The following are the parameters of MK-AZZJ4C for your reference:
1. 4.3 inch 720 HD display TCP/IP Intercom system
2. Calling, intercom, unlocking, monitoring
3. High quality aluminium alloy frosted panel + high-end glass lens + sensor buttons
4. Digital camera, stable and clear image shooting
5. Night vision and bright light compensation function
6. Full duplex voice intercom, comparable to ordinary telephone intercom
7. Android system ,ARM CPU, TUYA App (support Android and IOS system)
8. LPDDR3 1 GB memory, standard EMMC 8 GB storage expandable
9. RJ45 standard ethernet interface all-digital design
10. Low power consumption technology solution, saving energy and long life span
11. Dimension:400*140*51(mm)
12. Install method : Wall/flush install (390*130*46 mm)
13. Image/video advertising playback (advertising can be customized)
14. Door opening:IC/ID card/ password/ face recognition/ mobile phone
Our company has been focusing on intercom system for 11 years, we can provide you with different systems, like IP, 2 wire and 4 wire system, and we are also OEM & ODM factory, support to customize your brand and function, Thank you very much for your interest in our products, if you need more detailed information, please feel free to contact me.
Smart Doorphone With Tuya,Tuya Smart Doorbell,Tuya Video Doorbell,Tuya Smart Video Doorbell
Zhuhai Mingke Electronics Technology Co., Ltd , https://www.zhmkdz-electronics.com