Exploring the host-pathogen interaction is an important method for studying the disciplines of microbiology and immunology. The characterization of the host cell will be altered upon phagocytosis of the pathogen. Using real-time PCR or ELISA techniques, the behavior associated with genetically encoded proteins is measured to eliminate pathogens or immune escape. Most studies have been performed in vitro using primary host cells or cell lines. The data obtained by this method can account for the expression of RNA or the amount of protein recovered in cell culture. Under experimental conditions, it is reasonable for all host cells to have the same number of pathogens. However, pathogen absorption using phagocytic cells is not synchronized. Therefore, host cells containing different numbers of pathogens result in protein biosynthesis induced by different pathogens. Therefore, we constructed a technique to apply immunofluorescence high-throughput assays to detect the number of pathogens associated with host cells. Combined with multicolor molecular staining, it is possible to analyze the infection status of the host cell population and the results of the parasitic load (the phenotype of the relevant host cell).
The connection of parasites to host cells is an important part of microbiology and immunology. With the development of the application of monoclonal antibodies and fluorescent labeling reagents, there are more and more studies on the variation of host cells in the process.
In fact, multichannel flow cytometry and fluorescence microscopy have been used in research to detect the relationship between parasites and host cells. Both of the above methods have advantages and disadvantages . Flow cytometry (FACS) is capable of detecting the phenotype of host cells harboring parasites, but is not accurate to the number of parasites in a single cell. Moreover, flow cytometry requires single cell preparation so that localization of the cells cannot be achieved. Although the accuracy of fluorescence microscopy is higher, host cells with parasites can indicate the expression of in situ surface markers. However, it is still difficult to achieve a high degree of objectiveness of the host cell parasitic load marker intensity. Therefore, a technique that combines single cell quantification with host cell phenotypic analysis is essential.
In the analysis section of the experiment, we used the TissueQuest and StrataQuest software from Austria's TissueGnostics, which is capable of both single-cell quantitative analysis and host cell phenotypic analysis. A novel high-throughput multicolor microscopic imaging technique based on the patented Dot-Finder algorithm was used to analyze in situ and in vitro macrophages infected with L. major. This method achieves a comprehensive analysis of nucleated cells possessing pathogens. The intensity of the fluorescently labeled marker can be quantified in situ on a single cell level compared to flow cytometry (FACS) and does not require the preparation of a cell suspension. In addition, TissueQuest software enables accurate back-reversal of experimental data from cells in the image. The feature of reverse retrospective can be used to detect in situ cell subpopulations and locate the location information of the gate cells. Again, this feature can be used as the final result constellation.
Three markers were used in this experiment: DAPI for nuclear staining markers, AF546 (CD11b) and Cy5 (F4/80) for the other two.
Complete software analysis by adjusting the following simple parameters:
a. Core size
b. Area
c. Gray value application Reverse backtracking feature Real-time observation of the created positive cell scatter plot. The cut-off value was determined based on cell size and staining intensity. The positive value of DAPI and the activity L.major in the cell were detected, and the DAPI channel was created using the ring mask function.
Intracellular pathogen detection in lymphatic gland
The figure above shows the nuclear detection and ring mask calculation functions of the TissueQuest software. Grayscale imagery was analyzed using StrataQuest software. Single-channel grayscale images were analyzed using TissueQuest software.
(A) Nuclear detection by parameter adjustment (B) Creating a ring mask (C) The yellow highlight in the ring mask is for automatically screening pathogens in the ROI.
The TissueFAXSi-plus device completes the scanning portion of the image
This device is used for single-channel image acquisition, and four channels for fluorescence detection (DAPI, FITC, Rhodamin, Cy5). Each image contains nearly 50-200 cells, and TissueFAXS can be automatically stitched. Nuclear testing was performed using the parameters of the TissueQuest software. The image of each fluorescent channel and multiple FOVs can be automatically acquired, collected and combined in the later stages.
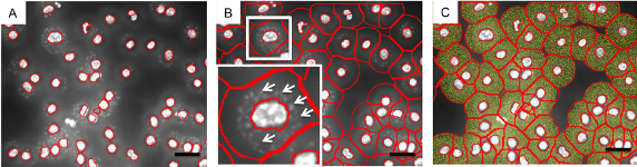
TissueQuest detects L.major pathogens in various ring masks
Based on the weak DAPI signals of active pathogens and complex background information, new algorithms need to identify pathogens from them. Each independent algorithm cannot meet all of the experimental needs at the same time. The Parasite finder preset program can choose the method to distinguish the background and the part to be analyzed according to the user's needs. (A) The ring mask encircles the cytoplasmic portion, representing macrophages in the absence of pathogen culture. (B) The blue portion is the ring mask of the infected macrophage, and the yellow arrow indicates the positive signal of DAPI. (C) The illustrated algorithm is used to detect weak signals that distinguish false signals in the ring mask of uninfected macrophages. (yellow part) (D) Create a false positive signal. Macrophages with more than 30 pathogens are marked in blue. (E) Detection of weak signals distinguishes non-pseudo-signals in the ring mask of uninfected macrophages. (F) All infected macrophages (yellow part and yellow arrow) were identified.
TissueQuest quantitative analysis of infected macrophage pathogen density and infection ratio
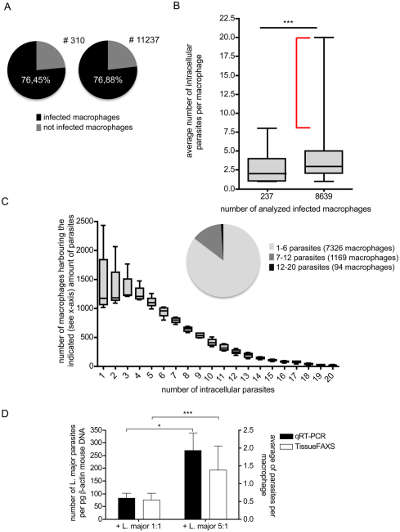
(A) Software automatically calculates infected and uninfected macrophages. (B) Quantitative analysis of the average number of intracellular parasites per macrophage. After analyzing 237 and 8639 cells, it was concluded that the samples selected immediately were sufficient to demonstrate the correctness of the total infection rate. (C) Relationship between intracellular parasites and macrophages. The number of intracellular pathogens and the macrophage corresponding to the pathogen corresponding to the Y-axis possess the number of pathogens.
This figure illustrates the high degree of heterogeneity of live pathogens in macrophages. The reason is speculated for diversified phagocytosis and enhancement of pathogen reproduction. The phenotype of macrophages determines the possibility of intracellular pathogen expansion.
Quantitative analysis of marker expression of bone marrow cells using infected and uninfected orthotopic macrophages
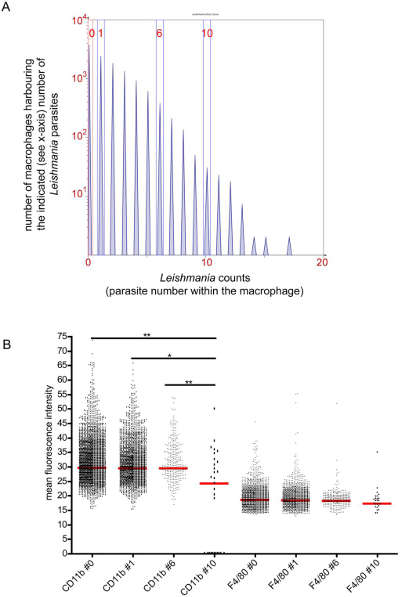
The TissueQuest software estimates each nucleated in situ host cell based on intracellular pathogens. The expression of different markers was compared by macrophages with different numbers of pathogens.
Phenotypic analysis of macrophages with different numbers of parasites.
(A) Histograms indicate the number of pathogens and Y-axis pathogens possessed by X-axis macrophages. TissueQuest software obtains the expression of the pathogens in the nucleus, ring mask, ring mask, CD11b or F4/80 in the ring mask (B) the average intensity analysis of macrophages with different numbers of pathogens.
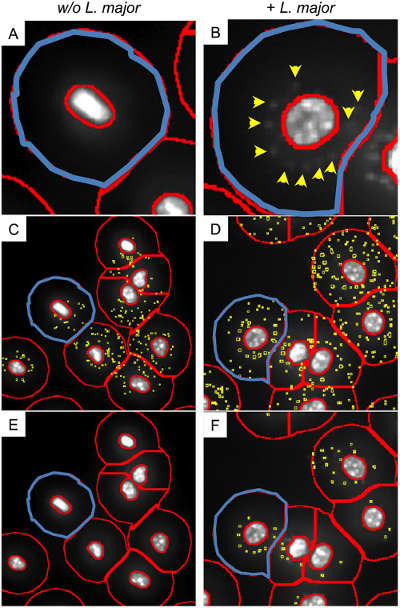
Diagnosis of in situ survival and inactivation of intracellular pathogens in vitro
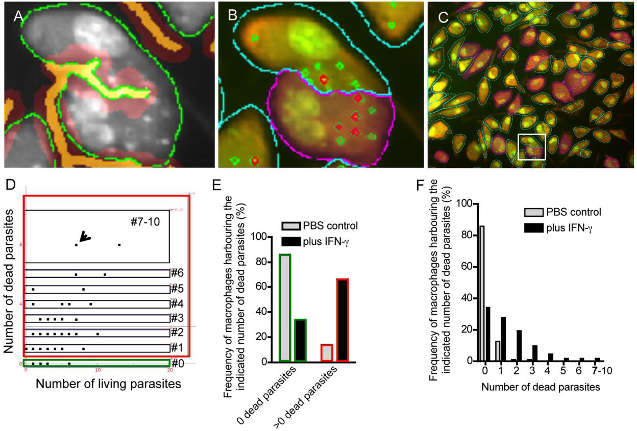
This figure is a quantitative analysis of living cells and dead cells in vitro. Because inactivated pathogens are unable to absorb DAPI, the software is able to make a difference in pathogens in the host cell. The samples were subjected to EB-AO parallel staining. StrataQuest identifies the cytoplasm (green part) of the cell and the cell-to-cell contact (orange).
(A) AO gray scale map for film detection (green portion), cell edge determination (orange portion). (B) Detailed analysis of adherent cells. (C) Reverse retrospective determination of macrophages possessing more than 2 inactivated parasites. (E) Frequency analysis of macrophages with inactivated parasites. (F) Frequency analysis of phagocytic cells with different numbers of inactivated parasites.
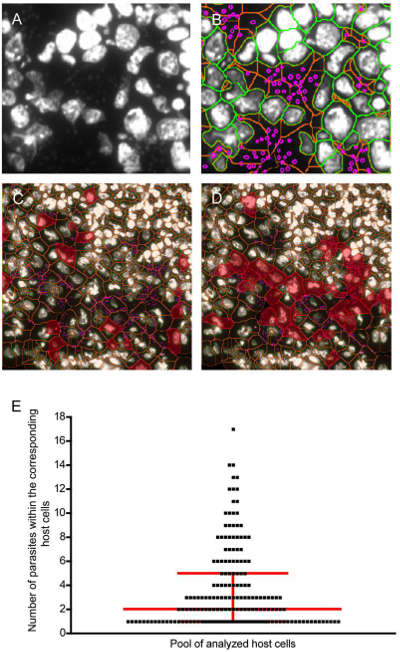
Frozen sections were analyzed using DAPI staining followed by TissueQuest. The DAPI signal indicates that the DNA of the pathogen has not degraded. Biopsy, biopsy, and pathogens before drying are still active. StrataQuest software creates nuclei (orange) and nuclear membranes (green). Thus, a DAPI positive pathogen can be identified in the cytoplasmic portion of the host cell.
This figure is an evaluation of the parasitic load. (A) Infected lymph glands. The gray scale map is DAPI + host cell nucleus and parasite DNA. (B) Overlapping images of the nucleus, cytoplasm and parasites (C) (D) Reverse retrospectively the parasitic load of cells infected with 3 and 5-10 parasites (E).
In this experiment, the TissueQuest and StrataQuest software were able to detect the following features:
1) Parasitic burden
2) Infected cell phenotype
3) Positioning
4) Cell-cell contact
Subsequent studies will focus on the molecular response mechanism of host-parasite linkages, and the improvement of disease cures in the microbial direction.
Organic stevia extract is a natural sweetener extracted from the pure leaves of the stevia plant. Stevia extract is a natural sweetener and a natural substitute for artificial sweeteners such as aspartame, saccharin, and sucralose. They are all zero-calorie sweeteners, but stevia is a natural source and has no industrial residues. The main components of stevia extract are stevioside and rebaudioside A. The sweetness is high, and the sweetness is 90-450 times of sucrose / edible sugar. It is ideal for patients with diabetes, hypertension, dental caries, and arteriosclerosis.
Stevia Extract,Organic Stevia Sugar,Organic Stevia Extract,Stevia Extract Powder
Organicway (xi'an) Food Ingredients Inc. , https://www.organic-powders.com