The overall survival rate is 97.3%, and childhood leukemia is expected to usher in new cell therapy
March 15, 2018 Source: WuXi PharmaTech
Window._bd_share_config={ "common":{ "bdSnsKey":{ },"bdText":"","bdMini":"2","bdMiniList":false,"bdPic":"","bdStyle":" 0","bdSize":"16"},"share":{ }};with(document)0[(getElementsByTagName('head')[0]||body).appendChild(createElement('script')) .src='http://bdimg.share.baidu.com/static/api/js/share.js?v=89860593.js?cdnversion='+~(-new Date()/36e5)];Recently, Bellicum Pharmaceuticals, a cancer immunotherapy in Houston, announced that its innovative cell therapy BPX-501 has achieved lasting gratifying effects in children with acute myeloid leukemia (AML) and primary immunodeficiency (PID). .

BPX-501 is a helper T cell therapy. In leukemia and immunodeficiency patients, a common treatment is allogeneic hematopoietic stem cell transplantation. BPX-501 can be injected into patients after transplantation, accelerate the reconstruction of the immune system, enhance the control of viral infection, and strengthen the effect of graft anti-leukemia without increasing the risk of graft versus host. Its CaspaCIDe security lock design couples the signal domain of Caspase-9 with a "chemically induced dimerization" (CID) binding domain. If the patient has serious side effects, doctors can use the drug rimiducid to activate the safety lock, allowing these cells to inject into the patient's T cells to undergo apoptosis, thereby regulating safety.
In a clinical trial called BP-004, the researchers evaluated the effects of BPX-501. They enrolled 38 children with AML who underwent haploid hematopoietic stem cell transplantation supplemented with BPX-501. At a median follow-up of 1 year, the researchers found that the patient's recurrence-free survival rate was 91.5% and the overall survival rate was 97.3%. Compared with the historical data showing 60%-80%, it is undoubtedly a huge improvement.
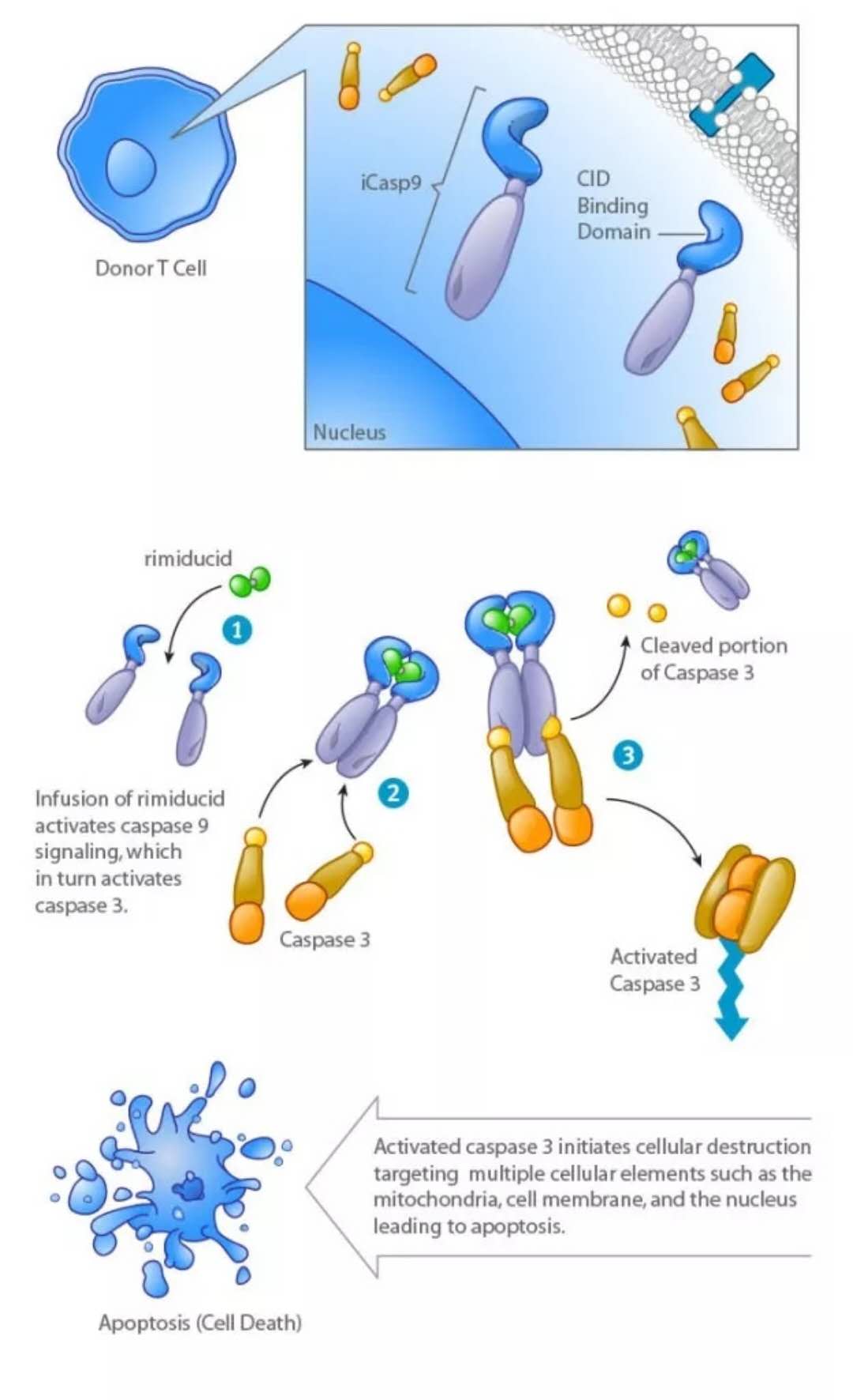
â–²CaspaCIDe security lock design (Source: Bellicum Pharmaceuticals official website)
Also in this trial, the researchers found that BPX-501 also resulted in high disease-free survival and overall survival in children with PID. Of the 59 patients, the researchers underwent a median follow-up of 1 year and found a disease-free survival rate of 88.1% and a overall survival rate of 88.6%.
"Recurrence of cancer is a serious risk for AML patients after receiving stem cell transplantation. The excellent results of children with AML indicate that BPX-501 is administered after haploid hematopoietic stem cell transplantation, which can effectively reduce residual cancer cells," BP-004 Researcher Dr. Neena Kapoor, head of the Hematology and Bone Marrow Transplant Program at Los Angeles Children's Hospital, said: "The delay in immune reconstitution can lead to serious infection complications, which is the leading cause of death in PID patients receiving haploid hematopoietic stem cell transplantation. One of the BPX-501 T cells infusion after transplantation can help the immune system to recover. The CaspaCIDe security lock can also launch a safety net to prevent graft-to-host risk from donor T cells."
With the support of these data, Bellicum looks forward to working with researchers and the FDA to discuss the registration of the next clinical trial. It is estimated that this trial is expected to begin in late 2018. We look forward to hearing more good news about this innovative therapy.
Reference materials:
[1] Bellicum Announces Interim Results Showing Low Rates of Cancer Recurrence in Pediatric AML Patients Treated with BPX-501
[2] Bellicum Pharmaceuticals Official Website
Nucleic Acid Extraction System
Nucleic Acid Extraction Kit,Nucleic Acid Purification Kit,Nucleic Acid Extraction System,Nucleic Acid Extraction Instrument
Hangzhou DIAN Biotechnology Co., Ltd. , https://www.dianbiotech.com