With annual revenue of 140 million yuan and net profit of 17 million yuan, such profitability is good in the medical device industry , but the equity of such a company has to be sold.
On May 21, the listed company Dong'e Ejiao (Dong A Ejiao Co., Ltd.) issued the "Announcement on Transferring the Equity of Dong'e Ejiao Ahua Medical Devices Co., Ltd.", stating that according to the company's development strategy of focusing on blood, nourishing and health care business It is proposed to transfer 36% of the shares of Dong A Ejiao Huahua Medical Devices Co., Ltd. (hereinafter referred to as "Ahua Medical Devices"), with a transfer amount of 295.558 million yuan.
It’s not bad assets to sell.
It is reported that Ahua Medical Devices was founded in 1987 and reorganized by Dong'e Ejiao in April 2000. The newly established company has a registered capital of RMB 3,333,300 and the legal representative is Qin Yufeng, Chairman of Dong'e Ejiao. At present, the company's main business is the production and sales of glass thermometers, electronic thermometers, electronic sphygmomanometers , blood glucose meters, medical plasters, medical adhesive plasters, Dongjia Ejiao holds 36%.
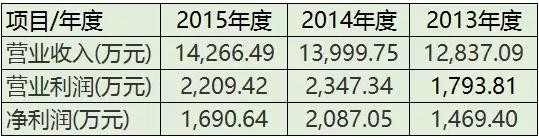
According to the announcement, the operating income of Ahua Medical Devices in 2015 was 1.4266 billion yuan, the operating profit was 22.09 million yuan, the net profit was 16.91 million yuan; the total assets were 100 million yuan, the total liabilities were 64.08 million yuan, and the net assets were 37.14 million yuan.
From the financial data of 2014 and 2013, the company's revenue status is also good.
Among the main products of Iowa Medical Devices, including thermometers. The company is also one of the leading manufacturers of thermometers in China. Its “Dongyue†brand thermometer has been rated as “Top Ten Brands of Thermometers in 2015â€, and is juxtaposed with Omron, Yuyue and Citizen.
According to the official website, the company is the chairman unit of the Glass Thermometer Professional Committee of China Medical Device Industry Association. The production and sales volume of glass thermometers ranks first in China and abroad. The mercury-free thermometer is a patented product developed by the company. It is the first domestic manufacturer to obtain a medical device production license, and its technical level is leading domestically. The development of fast electronic thermometers has made breakthroughs and achieved temperature measurement for 10 seconds.
In addition, the “Ahua†brand éºé¦™å£®éª¨è†, joint painkiller and other products developed and produced by the company have been sold to more than 20 provinces and cities such as Shandong, Beijing and Liaoning since they were put on the market.
This is a medical device manufacturer with best-selling products and strong profitability.
Nucleic Acid (DNA/RNA) Extraction Kit
1. Introduction
The total viral nucleic acid extraction kit is suitable for extracting total viral nucleic acid from serum, plasma, tissue homogenate and other samples. The kit is based on silica column purification technology, which eliminates the need for toxic phenol-chloroform extraction and time-consuming alcohol precipitation. This product has successfully extracted nucleic acids from hepatitis B A/C, hepatitis C, and norovirus standard. The obtained DNA/RNA can be directly used in a series of downstream experiments such as PCR, RT-PCR, and LAMP.
Notice:
1. The carrier RNA solid must be dissolved in Nuclease Free Water to 1µg/µl before use, and vortex to dissolve. Store in aliquots at -70°C. If you need to store it at -20℃ for a long time, please repackage it according to the number of times of use.
2. Dissolve Proteinase K (20mg/ml): Add Proteinase Dissolve Buffer to dissolve Proteinase K to a final concentration of 20mg/ml. Proteinase K dry powder can be stored at 2-8°C for one year, but dissolved Proteinase K must be stored in aliquots at -20°C. Repeated freezing and thawing of Proteinase K can affect its activity.
3. Buffer VHB must be diluted with 14 ml absolute ethanol before use and stored at room temperature.
4. Buffer RW2 must be diluted with 80 ml of absolute ethanol before use and stored at room temperature.
3. Shelf life
Except for Proteinase K and Carrier RNA, other components of this product can be stored at room temperature (15-25°C) for 12 months, and should be stored at 2-8°C for long-term storage. Proteinase K and Carrier RNA dry powder are transported at room temperature. Please store at -20°C after receiving the test product, and store at -20°C after dissolving.
Nucleic Acid Extraction Reagent Kit,Nucleic Acid Extraction Kit,Covid-19 Nucleic Acid Extraction Reagents,Nucleic Acid Test Kits
Jilin Sinoscience Technology Co. LTD , https://www.jilinsinoscience.com