Release date: 2018-02-12
Recently, the US media said that according to doctors, a second patient in California was treated in a historic genetic editing study, and about three months ago, the first patient to receive treatment had not yet experienced significant Side effects or safety issues.
According to the Associated Press reported on February 6, genetic editing is a more precise method of gene therapy, its purpose is to permanently change someone's DNA in an attempt to treat a disease.
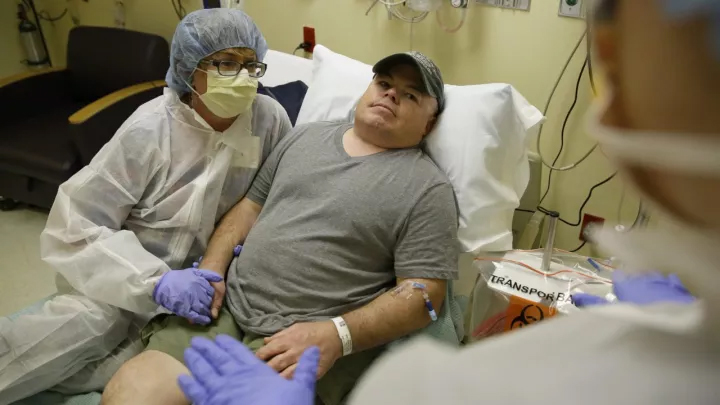
Brian Madd, who started genetic editing in November last year, is the first person to receive this method to treat Hunter's syndrome.
In November last year, 44-year-old Brian Mard became the first person to receive genetic editing to treat the metabolic disease mucopolysaccharidosis type II (also known as Hunter's syndrome). Hunter's syndrome is caused by a bad gene. Through intravenous drip, he received multiple copies of a corrective gene and a genetic tool to place the gene in the exact location of his DNA.
Paul Hamats, a doctor at the University of California, San Francisco, Benioff Children's Hospital, treated two people with the same disease. He said: "His situation is good, so we were approved to treat the second patient. The second patient is also in good condition."
At a medical conference in San Diego, Hamats reported on the safety of Mard during the first six weeks of treatment. Sangammo Pharmaceuticals, which manufactures the gene editing tool "Zinc Finger Nuclease," is testing the tool for two metabolic diseases and hemophilia, a bleeding disorder.
The company said that more safety information and preliminary results related to effectiveness should be obtained in the middle of this year.
Hamats said that after 4 days of treatment, Mad had dizziness, cold sweats and weakness, but these conditions disappeared after one day. Mad has also had severe cough and partial collapse of the lungs, but doctors believe that these are not related to gene therapy because he has had similar problems before.
According to the report, it is important that his liver has no signs of damage.
Hamats said, "That is the most worrying part," because changes in the liver may mean that the immune system is fighting the therapy and may undermine its effectiveness.
The report said that the results of the liver were welcome news, and other recent reports have caused panic. James Wilson, a well-known gene therapy scientist from the University of Pennsylvania, published two studies reporting liver problems and other serious problems in monkeys and piglets undergoing experimental gene therapy. Some animals have to be euthanized.
These animal studies tested the practice of injecting higher therapeutic doses intravenously. The relevant agent uses a virus to bring the gene into the cell. This virus-related virus is widely used in human gene therapy. But Wilson said he believes that the results of animal studies are not related to dose, different types of virus or different modes of administration.
These results may mean that it would be difficult to develop gene therapy for some neuromuscular diseases - the use of higher doses in animal studies is considered necessary to allow the drug to enter the brain and spread throughout the muscles.
The study conducted by Madd's Sangammo company used a different kind of virus and the dose was much smaller.
Wilson said that publishing all security concerns as soon as possible is critical to the field. In 1999, he helped lead an early gene therapy test, but this led to the death of a young man, leaving other studies on hold for years.
An editorial in Human Gene Therapy says that gene therapy should not stop because it may deprive patients of the opportunity to receive treatment and live. The magazine published an animal study from Wilson. Last year, the United States approved the first gene therapy to treat cancer and a hereditary blindness.
Source: Associated Press
Processing high-throughput samples, intelligent reuse for large-capacity publishing, work surface: 200cm, 8 sample injection needles, 12 temperature-controlled incubation positions, 12 room temperature incubation positions, 32 plate storage positions, Sunrise microplate reader, HydroFlex plate washer, up to 512 specimens, sequential loading of samples, reagents, microplates Parallel loading of up to 6 plates for fast dispensing.
The automatic enzyme immunoassay analyzer is based on the principle that the enzyme and the substrate can produce a color reaction, the absorption lines of different substances have different characteristics, and strictly abide by the Lambert-Beer law, quantitative and qualitative analysis of substances. instrument. The method of analyzing the content of various enzymes such as antigen or antibody generally mainly adopts colorimetric method. In practice, spectrophotometry is the basic working principle of an automatic enzyme immunoassay analyzer. The light emitted by the light source lamp becomes a beam of monochromatic light after passing through a filter or a monochromator. The monochromatic light beam passes through the sample to be tested in the microtiter plate, and part of the monochromatic light beam is absorbed by the sample and reaches the photodetector. The intensity of the light signal projected on it is converted into the magnitude of the electrical signal by the photodetector. This electrical signal is processed by pre-amplification, logarithmic amplification, analog-to-digital conversion, etc., and then sent to the microprocessor for data processing and calculation, and the test results are output by the display and printer. The microprocessor completes the movement in the X and Y directions of the mechanical drive through the control circuit.
The automatic enzyme immunoassay analyzer adds the sample to the microwells of the pre-coated antigen or antibody microtiter plate, washes after the reaction, removes the unseparated ligand, then adds the enzyme isolate, after incubation, washes again , remove the unseparated compound, and then add the enzyme substrate, after the reaction, the colored final product is formed, and the stop solution is added to stop the reaction. The absorbance of each microwell of the microtiter plate is read by the wavelength that has been set by the spectrophotometer. The concentration value of the analyte in the sample is calculated by the absorbance value of the sample and the standard curve, so that the quantitative result can be obtained, or the absorbance of the sample is compared with that of the standard product, so that the positive or negative qualitative result can be obtained.
ELISA analyzer,enzyme immunoassay workstation,automatic enzyme immunoassay analyzer,automatic enzyme immunoassay system
Jilin Sinoscience Technology Co. LTD , https://www.jilinsinoscience.com