The State Council Legislative Affairs Office and the State Administration of Cereals and Drugs jointly promulgated the newly revised "Regulations on the Management of Medical Devices." It is clear that the principle of risk classification from low to high should be applied to the medical device's strict and separate supervision and control principles. The new version of the Regulations on the Administration of Medical Devices is scheduled to be implemented on June 1.
The most significant change in the new version of the "Regulations on the Management of Medical Devices" is that medical devices are classified into one, two, and three categories of risks from low to high, and that one category of medical device products is changed from registration to record management; the second is the right to approve medical device registrations. Decentralization to the provincial food and drug regulatory authorities; three types of medical devices such as disposable sterile syringes, pacemakers and other high safety risks, strict registration supervision by the State Food and Drug Administration, but also to strengthen the monitoring system of adverse events after the listing Reevaluation system and recall system.
The new “Regulations†stipulates that a unified information platform for medical device supervision shall be established throughout the country, and the food and drug regulatory authorities shall timely publish medical device licenses, record filings, spot checks, and investigation and investigation of illegal activities through the platform. In addition, the new “Regulations†has added a complaint reporting system and rewarded confirmed reports.
The new "Regulations" intensify penalties for violations of law and regulations. For example, the implementation of heavy penalties for the production and operation of medical devices without permission imposes a fine of up to 20 times the value of the goods.
Focus
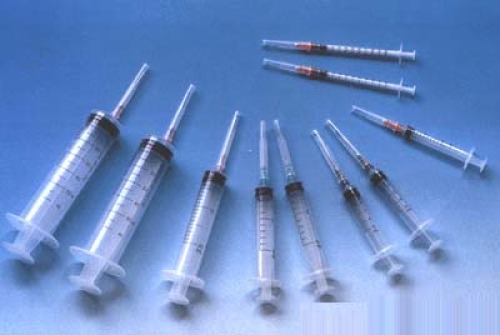
Disposable needle management requires license management
Wang Zhenjiang, Director of the Department of Legal Affairs of the State Council Legislative Affairs Office, explained the classification supervision: “pressurizing†high-risk product production and operation companies and “relaxing†low-risk product production and operation companies.
Specifically, the new edition of the Regulations has new changes in the classification and supervision of medical devices in both product registration and business operations. During the registration process, the first category of medical devices was implemented to implement product registration management, the second category was implemented by the provincial food and drug regulatory department to implement product registration management, and the third category was implemented by the National Food and Drug Administration to implement product registration management; The management of the first type of medical device is neither permitted nor implemented. The management of the management of the second type of medical devices is implemented, and the license management of the third type of medical devices such as disposable sterile syringes is implemented.
â– Links
Medical Devices in Three Types of Supervision
The first category: Medical devices that can ensure the safety and effectiveness through routine management, such as medical beds, basic surgical scissors, forceps, and tweezers.
The second category: medical devices whose product mechanism has been recognized internationally and domestically, mature technology, safety and effectiveness must be controlled, such as thermometers, sphygmomanometers, stethoscopes, clinical testing instruments, electrocardiographs, etc.
The third category: medical devices that are implanted into the human body, or used for life support, or have complex technical structures, are potentially dangerous to the human body, and must be strictly controlled in terms of safety and effectiveness, such as disposable sterile syringes, and disposables. Inoculum, pacemaker, blood purification equipment, artificial organs, etc.
Ankle Surgical System
Medton Medical , https://www.medton.cn