In 2018 , many companies were reported by real names, triggering large-scale national flight inspections. During the inspection process, the company exposed serious “concealed data†behaviors. At the same time, it also exposed many problems such as the daily management level and the insufficiency of the quality system.
Recently , the Ministry of Justice once again sent a revised "Drug Administration Law Amendment (Draft for Review)" to the Chinese Pharmaceutical Association in the form of "extraordinary" to solicit a letter of request. The cancellation of GMP certification appeared in the fourteenth revision of this revision. Article, the elimination of GMP certification does not mean that the threshold for the production of pharmaceutical companies is reduced. On the contrary, pharmaceutical companies will face more stringent inspections, especially the flight inspections that are not informed in advance will be more normal.
Overview of drug GMP flight inspection
Drug GMP flight inspection is a form of follow-up inspection of pharmaceutical production enterprises. It refers to on-site inspections that are not notified to the inspection department beforehand. The key inspection targets are drug manufacturers suspected of violating drug GMP or having bad behavior records. In addition, the state The Food and Drug Administration can also include the production of key products such as sterile drugs into the scope of flight inspection according to the needs of supervision, which is an important means to ensure that enterprises strictly follow the production of pharmaceutical GMP .
According to the "2017 National Drug GMP certificate to recover Statistics of" show, the year 2017, 31 provinces and cities nationwide were recovered drug GMP certificate 157, then back to the drug GMP certificate 64. Once the GMP certificate is withdrawn, it is not easy to get it again.
Differences and characteristics between flight inspection and GMP certification inspection
In the traditional GMP certification method, the biggest difference from the flight inspection is to determine the inspection variety and scope in advance, to pre-notify the notification, set the time, and set the route. The enterprise can fully prepare the various archives for inspection. The regulatory authorities see the enterprise carefully. The state of preparation, rather than the regularization state, many problems cannot be effectively discovered.
Compared with the traditional GMP inspection, the characteristics of the flight inspection have five very prominent features, one is the confidentiality of the action, the second is the suddenness of the inspection, the third is the insulation of the reception, the fourth is the flexibility of the scene, and the fifth is the record. Immediacy. Being able to understand the real operating status of the company being inspected and the true level of production quality management can more effectively identify the problems existing in the enterprise, supervise its improvement, strengthen the self-discipline awareness and law-abiding consciousness of the enterprise, and there will be major safety hazards and counterfeiting. Counterfeit drugs are killed in the cradle.
Main problems exposed during flight inspection
According to relevant statistics, during the period from 2016 to 2018 , about 233 companies in the national inspection of the State Food and Drug Administration and the provincial food and drug administrations were found to have problems. On the whole, the problems of pharmaceutical manufacturers are as follows. Aspects: quality management, supplier management, verification management, organization and personnel, document management, materials and products, equipment, plant and facilities, production management, process prescriptions, etc. , according to the defects of the drug inspection of the National Bureau from January to August 2018 According to the project statistics, it can be seen that the most prominent and universal problems are concentrated in quality management, with an overall proportion of over 40% .
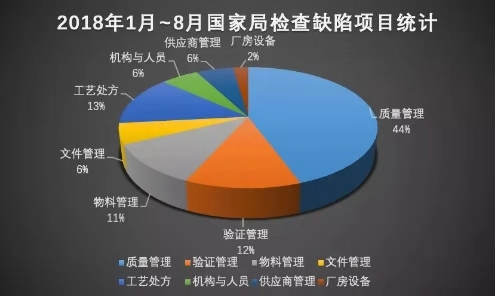
The most prominent problem in quality management is in terms of data reliability, with an overall proportion of more than 50% .
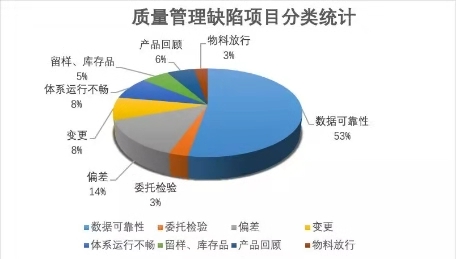
The main problems of quality management are:
1. Drug sampling and inspection operations do not meet the requirements and are not implemented in accordance with the SOP;
2. Sample management and reference product and reagent test solution management do not meet the requirements;
3. The change implementation record is incomplete, the deviation investigation is not thorough, and there is no CAPA measure; no annual product review is conducted;
4. The more serious problem is that the quality system cannot operate effectively and the laboratory management is not standardized.
Among them, the problem of data reliability mainly shows:
1. In terms of computerized system rights management, the rights are not set so that anyone can enter the system, or the irrational rights are not matched with the privilege, and the operator has the right to delete and modify the data;
2. The audit trail function is missing and cannot be traced;
3. Unable to find the original data information, data fraud, record fraud: after the computer time is modified, the stability test sample test data is forged, and the record is compiled.
How to deal with the flight inspection
All certification inspections include flight inspections. It is carried out in terms of people, machines, materials, laws, and loops. The normal operation of enterprises is also in these aspects. How to deal with the flight inspections of surprise attacks is the most important thing in daily work. In compliance with the regular training of employees and enhance their quality awareness, laboratory informationization can be enforced according to standard procedures and standard SOPs through its specific functions, ensuring continuous daily compliance, and must have the function to solve the above problems. The characteristics are as follows:
1. Pay attention to the assignment of role rights, and be able to meet the role permissions assigned to their needs according to the user's responsibilities;
2. Strict system password security restrictions are required. There are restrictions on the complexity of the password, the length of the password, and the validity period of the password. When the password is valid, the system will reject the user login.
3. Must have complete audit trail function, set up perfect electronic signature for any operation , record data creation, modification, deletion, etc. (including operator, operation reason and operation timestamp, etc.); safeguard audit trail verification Smooth development, auditing operations and data, tracking data generation, review, and modification processes to achieve traceability of operations and data;
4. It is necessary to provide a stability inspection module . From the establishment of the stability scheme, the stability investigation plan triggers, the sample, the sampling, the sample, and the product inspection are all completed in the system. All the time information is the server time, and the experiment personnel are eliminated. Modify computer time to forge stability test sample test data.
5. Need to provide the laboratory with inventory management module for traceable inventory transfer details , which can accurately manage the test consumables (including reagent test solutions, reference products, standards, etc.) (including distribution, requisition, profit and loss, destruction). Processing, etc.);
6. Need to provide the laboratory with a sample management module that can view the complete life cycle , and can develop standard workflow management according to business needs. The sample must be executed according to the standard workflow from the start of the inspection.
7. It can automatically control the change of sample state value according to strict logic rationality, and realize very precise control of various operations of samples, including trace sample and sample product use.
8. A fully validated SOP implementation platform is required to ensure that laboratory personnel can only perform inspections according to quality standards, automate laboratory data collection and record reporting, and eliminate data and record fraud;
ChuangTeng Technology is a well-known high-tech enterprise providing comprehensive research and development, process testing, production information platform and consulting services for life science and materials science. The laboratory informationization solution (LIMS+LES) provided by ChuangTeng Technology integrates samples, personnel, instruments, inventory, data, documents, events, record reports and other modules to fully meet the above functional requirements, of which LES As the only fully validated SOP execution platform, mandatory personnel are enforced according to established standards, fully guaranteeing data integrity in process execution ; making post-supervision monitoring into incident monitoring, improving the standardization of laboratory operations, and making substandard Operation becomes unworkable, thus ensuring daily compliance, so that companies are not afraid of "flying inspection . "
Certeng Technology's verification and implementation team members are from well-known domestic and foreign pharmaceutical companies and laboratories, through relevant computer verification training; to ensure that our systems are in compliance with the "Good Manufacturing Practice Guide" GAMP5 and ISO 17025 requirements; Verification of implementation experience, rigorous testing and verification, safeguarding the system's own regulatory compliance, eliminating data fraud, fully complying with the special regulatory background of pharmaceutical companies, meeting GxP and FDA 21 CFR Part 11 and other relevant regulatory requirements.
Radial Artery Compression Devices
Radial artery compression devices, also known as radial artery compression devices or wristbands, are used to achieve hemostasis after a transradial cardiac catheterization procedure. The device is applied to the wrist and inflated to compress the radial artery, which is the artery that supplies blood to the hand and fingers. This compression helps to prevent bleeding and hematoma formation at the site of the catheterization.
Radial artery compression devices are preferred over traditional compression methods such as manual compression because they are more effective, comfortable for the patient, and allow for earlier ambulation and discharge. They also reduce the risk of complications such as radial artery occlusion and nerve injury.
In addition to cardiac catheterization procedures, radial artery compression devices may also be used after other procedures that involve the radial artery, such as transradial access for arterial blood gas analysis or for the placement of intra-arterial lines.
Radial Artery Compression Devices,Outlet Radial Artery Compression Device,Disposable Radial Artery Compression Device,Medical Consumables Tourniquet
Changzhou Weipu Medical Devices Co., Ltd. , https://www.wmlaparoscopic.com