
Clinical research needs to produce results, grades, and high quality, which cannot be separated from clinical data.
The clinical research database is different from the business management software such as "doctoral system" and "electronic medical record system". It is the flexible application of clinical data from four aspects: entry, find, see, and exit. The more the total amount of case collection, the more complete the information, the more accurate the description, the larger the time span, the wider the distribution range, and the higher the sample collection density, the higher the value of the database.
The construction of the scientific research database is supposed to be responsible for the hospital management and the information department (or network center, computer center). However, many hospitals give priority to the construction of business systems, and the construction of scientific research databases is lagging behind, and the investment is very small. The main reason is that the construction output of the research database is not timely, and it is impossible to make immediate achievements. Even if it is a result, it is generally attributed to the clinical researcher. It is difficult for the management who does not participate in the research to benefit from it. Even if some hospitals have established research database systems, they can only basically meet the needs of the commonality of departments, and can not meet the individual needs of clinical departments for their own research. Some specialists also have multiple study groups or sub-disciplines, and there are more detailed differences in case collection requirements.
Since waiting for the hospital to establish a scientific research database is nowhere in sight, and the research database established by the hospital can not meet the needs of the specialist, then the department can only find a way to establish the database it needs.
Since procurement is originally the responsibility of management, the procurement of research databases may involve multiple departmental coordination. Therefore, the clinical department encounters various problems in building a database system:
1. Project approval and budget quota (funding issues)
Marching in battle, grain and grass first. Purchasing a database always requires a fee unless it is developed by itself.
There are two kinds of research and development on their own: one is to ask the technical staff of the hospital information department to develop, and the other is to arrange the postgraduate research and development of the computer. The problem of self-development is that it is not professional enough, and the enthusiasm is not high, the motivation is insufficient, and the output is very sorrowful, and often it will end in vain.
A source of funding is required for the procurement database. Since it is a departmental behavior, hospitals rarely pay for this separately. Almost no department head is willing to spend the "small treasury" money, although they do not admit that there is a "small treasury." A wide range of department heads can often find third-party cooperative companies such as medicines, equipment, consumables manufacturers and charities to solve the cost problem. However, they always owe each other a person, and not every manufacturer has a long-term vision and dedication.
Therefore, the best way is to set up a project to declare the problem, and solve the problem of cost. Every year, our country has a large amount of research budget, and if the clinical departments that really want to do research are not going to fight for it, they will be cheaper and those who are opportunistic will be able to consume the budget. Therefore, applying for a project is not only a positive response to the national research policy, but also a great respect and best return on research funding.
Although many of the affiliated hospitals have a large number of subject funding support, most of them focus on the mouse experiments, reagents, consumables, specimens, equipment, travel and other expenses required for the research routine. Without realizing the importance of the database, it is rare to consider the construction cost of the database at the beginning of the project. It is almost impossible to pay for the database technical service fee from the expense afterwards.
Even some researchers have considered the technical service fee at the beginning of the project application, but they will also encounter negation or substantial reduction of their own level by a certain leader who is “unfamiliar†or “too knowledgeableâ€. Some projects can't even be submitted to the approval stage at all, and they are directly squeezed out by other more “non-upper†projects.
In fact, whether the project is important or not depends on the topic itself, but on the distribution of the interests of the project. The person who approves from the clinical department to the medical department, from the president to the principal, from the hospital to the science committee, the health bureau, etc. may have more than one level, then the interest of the subject must be at least beneficial to him in the long run. It is best to have a short-term interest that is highly relevant to him and to facilitate the passage of the project.
To this end, we recommend that the management with the desired interests be included in the group members as much as possible without affecting the direction of the subject. If there are academicians, directors, members of the discipline committee, dean, etc., you can try not to miss it. This hospital does not have it. Other hospitals can do the same. They hang a title and do research themselves. There is no harm in the achievement of one signature. When the benefits are transferred, the risks are diluted and evenly distributed. More importantly, the cycle may be shortened and the cost support will be improved. We have established a database collaboration platform for a certain disease area. At that time, their basic budget was only 600,000 yuan, and the approval process was basically impossible. Later, we helped to improve the technical part of the program. The software and hardware costs of data construction and the labor costs of uploading data by the collaborating unit (paying 10 yuan for each case) were budgeted, and the cost reached 1 million. Then the director of the Health Bureau was invited to the members of the research team. As a result, not only did the project pass the examination and approval, but the budget was raised to 1.2 million yuan.
2, database and hospital system interface problems (interface problems)
Hospital business systems such as electronic medical records systems, clinical pathway systems, and medical order systems are generally responsible for procurement, construction, and maintenance of the information department (or network center). This is their duty and their scope of power. Therefore, if the department establishes its own database, it avoids the direct involvement of the Information Section and reduces their workload and liability risks. However, in order to achieve a large number of research databases, it is necessary to debug the HIS interface by considering the case data imported into the department from the clinical business system. The interface first requires the Information Section to authorize and then requires them to provide relevant technical support. The Information Section has neither the responsibility nor the obligation to provide this service to the clinical department. Therefore, it will refuse to provide authorization and support on the grounds of network security. Some hospitals also uninstall or disable the U disk and CD of the internal network to ensure the security of the network system.
The current hospital business system is rarely taken over by a company. More and more systems (such as LIS, PACS, EMRS, NIS, etc.) are independently developed by different companies, and interfaces need to be used to exchange data between systems. The research databases that need to be connected to HIS are used on the internal network, and are not external. There is basically no data leakage or system instability. Therefore, the system interface is very common, and the experts in the information department know that there is no technical problem, just the problem that should be provided.
The clinical data was originally generated by the clinician's daily work and should be used for his own research. Because the hospital business system cannot support scientific research needs, it is inevitable to establish a separate scientific research database for rearrangement and flexible use. The Information Section and other departments belong to the auxiliary department, and the responsibility is to provide software and hardware technical services for the clinical departments. However, because of different angles, the Information Section does not pay much attention to clinical research, and it is impossible to understand the detailed needs of clinical research. This phenomenon is widespread, and smart people will take the initiative to make good relationships with people in the information department. It is necessary to handle the relationship with the director of the information department and to handle the relationship with the technician. Although this is not absolutely useful, there is no harm in the relationship.
HIS interface debugging mainly solves the problem of clinical data import. The main page of the medical record, diagnostic information, surgical information, inspection and inspection information, medical orders and cost information are all massive. It is neither necessary nor labor-intensive by manual input, and there is also the possibility of erroneous data. Importing through the interface can improve efficiency and quality assurance, and is more in line with the basic requirements of scientific research on the accuracy of data and massive information.
The best way is to find support from the higher authorities and strive for support from the authoritative leadership. For example, if the above-mentioned funds are approved, if the development of the project is indeed helpful to the overall strength of the hospital, then the Information Section will be more supportive and cooperative. "The method is always more than the problem" is the truth. Leaders attach great importance to the problem, and the problem will be solved quickly; if the leader pays attention to it, the problem will be solved slowly; if the leader does not pay attention, then the problem will not be solved. This is the national condition.
3, database finishing and maintenance issues (input problems)
In addition to the cost issue mentioned in the first point above is an economic investment problem, the input of energy is also a good problem in the construction of scientific research database.
The database of purchases is not valuable for purchase, nor is it valuable for importing clinical data through the HIS interface. It depends on continuous energy input and time commitment, so that it can be accumulated over time to enrich it and activate its value. "You don't manage money, money doesn't care about you" - the same is true for research databases. There are a lot of clinical data that need to be manually organized because of source limitations, different formats, or loss of damage. For example, the surgical photo data does not necessarily exist in the business system, and is collected by the operator himself. The follow-up data is obtained after the patient is discharged from the hospital for follow-up; some valuable elements in the electronic medical record document also need to be manually arranged. , enter the specialist indicator information.
Because clinicians are busy with the tube bed business every day and "related" to the business, there is hardly enough time to calm down the database. Therefore, conditional departments can recruit or arrange separate secretaries to be responsible for the use of the database. The departments that do not have separate manpower arrangements are only organized by the doctors themselves. For young doctors who are new to work, energy is not a problem, but an attitude problem. Those who realize that they have the right to speak in the future will understand the importance of the database. The collection of cases is not a one-off, but depends on the accumulation of time. Organize a little every day, and spend less time each day, and the output will be reflected in the day.
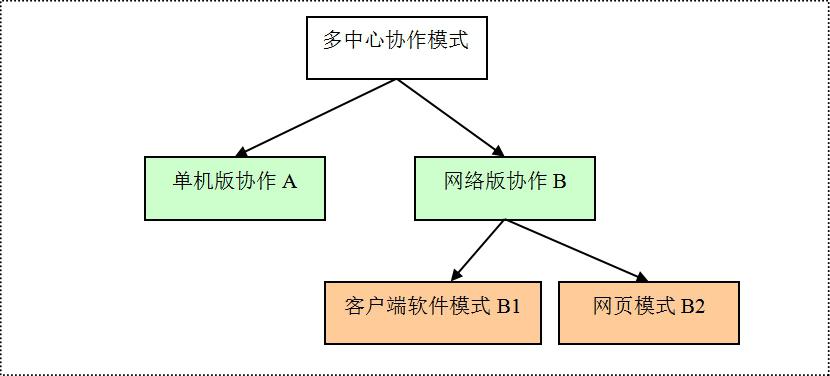
4, multi-center research database construction model problem (pattern problem)
Many disciplines have large-scale topics and important public relations directions, which often require multi-center collaboration to achieve better results. Multi-center research is first and foremost a data support issue.
How to better collect case data from collaborative units? Technically divided into two modes: [single version collaboration A] and [network version collaboration B].
The stand-alone version of collaboration A: is to install a stand-alone database system for each unit, docking the HIS system of each hospital, importing and sorting out their own clinical data, and then importing and exporting to realize the exchange of case data and data migration.
Collaboration B of the online version: is the collaborative unit online registration data and online upload of clinical data. It is divided into two modes: client software version [B1] and web version [B2]. The client version is like QQ software, and the web version is like an online email system (126 mailboxes, QQ mailboxes, Sina mailboxes, etc.).
The difference between stand-alone collaboration [A] and online collaboration [B] is:
1. The stand-alone version does not depend on the network, and can be copied and sent by the U disk after packaging. The web version relies on the web, but it can be implemented online in real time.
2. The stand-alone version can be connected to the HIS import data, and the network version does not support the docking HIS.
3. The stand-alone version can be input offline and input in different times; the web version needs to be input online, which is not convenient for frequent switching and divided input.
4, stand-alone version is suitable for deep collection, online version is suitable for streamlined collection.
5, stand-alone version of the data can be stored off-site, local retention. The online version of the data only exists in the central database.
6, encounter upgrades, stand-alone version requires manual upgrade, network version can be upgraded in the background.
7. The stand-alone version can help the collaborating unit to establish its own department database, and the online version can only contribute data.
8, stand-alone version needs to pay separately, but can buy. The online version needs to be activated by the terminal, open the account, or close at any time.
9. [Client Software Mode B1] in [Network Edition Collaboration B] has some of the functional advantages of [Single Machine Collaboration A] compared to [Web Page Mode B2]. The area [B1] is different from [A] in the depth and breadth of data collection. [A] mode can be more depth and breadth because it can be connected to HIS. And [B1] is all manually input, so the content collected should not be too deep, and the more streamlined the more effective.
How to choose?
If you need to collect case data in depth, choose stand-alone version A mode. Because the collaborating unit can install the stand-alone software on the intranet, you can connect the HIS to import clinical data.
Need to quickly collect (streamlined to: demographic data + specialist index data), all manually entered, do not dock HIS, and need to control the uploading of the collaboration unit (more than 100 cases do not upload to remind, more than 500 cases do not upload to limit registration, etc.) . Then choose the online version of B1 mode.
Only the collaborative unit contributes case data, and does not need to consider the multi-center research platform stored by the collaboration unit itself, and does not connect to HIS, and all rely on online one-time filling, and can adopt [web version B2 mode].
5, the version of the research database version problem (version problem)
According to the research direction, stage objectives, scope of application and cost budget, it can be divided into:
Departmental stand-alone version: build a stand-alone application with a desktop database engine. It can be collected simply or in depth to collect clinical data. You can dock HIS to import data.
Department LAN version: The database is installed on the LAN server, and the workstation computer is used for networking. You can dock HIS and store scientific research cases centrally. The server can purchase a dedicated server by itself, or use a desktop as a server, or apply for the server space of the Information Section.
Department WAN version: The database is installed on the WAN server. You can rent servers, cloud hosts, hosting, and more. The workstation computer must be connected to the external network and cannot be connected to the internal network. Therefore, HIS cannot be docked and can only be sorted by hand. Collecting content needs to be streamlined.
Single-Center Collaboration WAN Edition: Same deployment as the Department WAN version. After the patient has created the file, the patient information can be registered in any of the collaborating units. There shouldn't be too much content to focus on structured information. Online collaboration for regional purposes for ongoing follow-up. For example: maternal check-ups, child care, follow-up of chronic diseases, etc.
Multi-center collaboration WAN version: The sponsoring unit establishes a network version database, and the collaboration unit installs the client software on the external network computer, manually registers, and the content is streamlined, focusing on structured data. Registration is completed, bulk upload. Can be viewed through the web or client. Support clients to download their own data. Suitable for single-case database collaboration targeting case collection.
Multi-center collaboration stand-alone version: The sponsoring unit establishes a network version or a stand-alone version database. The collaboration unit installs the client software on the intranet computer, which can be connected to the HIS import, or can be manually registered, supplemented, rich in content, and supports surgical photos, video materials, etc. . Regularly export and send it to the sponsoring unit for data migration and summary. Initiators and collaborating units have their own separate databases. Suitable for multi-center collaboration where in-depth collection of case data is required.
6. How to ensure the total amount and quality of case collection (power problem)
If the department database is responsible for the department, it is necessary to consider the dynamics and responsibilities of the secretary. Salary bonus is one aspect, leading the secretary to publish relevant articles, and the result is also an aspect.
In general, multi-center research collaboration platforms are initiated by disciplinary units and invite brothers to participate in collaboration. The collaboration unit contributes data to the sponsoring unit, first of all [see the foreground, give face, and enthusiasm]. But relying on face is not enough. In particular, it is the director who promises to participate in the collaboration. The doctor or nurse is responsible for data entry. Involving the accumulation of time and perseverance, we need economic benefits to protect. Therefore, it is necessary to consider the labor costs of the cooperative unit in the project budget for the project. This labor service is implemented on the input person to meet the power problem.
The labor costs of the collaborating unit can be estimated based on the research cycle and the scope of case collection. For example, the project cycle is 3 years, and the monthly calculation is 36 months. It is expected that each collaborative unit will collect 100 cases per month, and the first stage will be 10 collaborative units. The total amount of data is: 100 cases * 36 months * 10 units = 36,000 cases. According to the 10 yuan case, the labor service fee is 300,000 yuan. If you follow the example of 20 yuan, then it is 720,000 yuan.
7, database software supplier selection problem (selection problem)
Since it is a procurement database, in addition to cost issues to consider, the choice of suppliers is also an important thing. Some people think that big companies are reliable. Of course, from the strength of the company. But the research database projects are tens of millions. Otherwise, tens of thousands and hundreds of thousands of projects will go to large companies, and often they will not receive too much attention. The task is arranged and returned to one or two technicians to take responsibility. Even with millions of big projects, big companies are unlikely to arrange the entire company to be responsible for delivery. Since it is a company employee, stability is questionable. The resignation of an employee or the substitution of a person in the middle has always had an impact on the project. Moreover, employees are mainly engaged in completing tasks, and their interest in new needs will not be too great.
Professionalism is very important. It is a long-term strategy to deeply understand the clinical needs and actively cooperate with the clinical upgrading of research and development.
Research funding is hard to come by. It is recommended to speak with results and see results.
List of veterinary drug raw materials
Veterinary medicine heat-clearing medicine: all the medicines that are cold and cool and can clear and relieve heat-related diseases are called heat-clearing medicines.
Heat-clearing medicine can be divided into the following categories: â‘ heat-clearing and lowering gunpowder: the main function of this type of medicine is to clear the qi and divide the real heat, which is suitable for reciting the inner heat that is caused by the high-heat fire.
Excessive, thirsty, fond of drinking, urination, short and red urination, cough and asthma due to lung heat, tongue sores, red color, yellow and dry glutinous rice and other symptoms. Our unit can provide gardenia extract, bile, knowledge
Mother, bitter and other extracts. â‘¡ Heat-clearing and damp-damping medicines: Most of these medicines are bitter and cold, bitter can dry dampness, and cold can clear heat, so they are used for red and yellow lips and thick tongue coating.
Greasy, damp-heat jaundice, short and red urination, damp-heat diarrhea, sore yellow swelling toxin embolism. Our unit can provide extracts such as Coptis chinensis, Scutellaria baicalensis, Treats, Yin Chen, Sophora Radix et Rhizoma etc. â‘¢ Clear heat
Detoxification drugs: This type of heat-clearing and detoxifying drugs are mainly used to clear away heat or fire poison caused by excessive heat. These diseases are mostly manifested as persistent high fever, sore throat, sore yellow furuncle, eye
List of veterinary drug raw materials,Buy Online Tylosin Phosphate Premix,Tylosin Phosphate Premix Powder,Bulk Tamoxifen Citrate Powder
Xi'an Henrikang Biotech Co.,Ltd , https://www.henruikangbio.com