Identification and structural analysis of potential migratory materials in food packaging paper and paperboard using the MS E function of the UPLC/Q-Tof liquefaction meter and the MassFragment software
Malcolm Driffield, 1 Antony Lloyd, 1 Emma Bradley, 1 Dominic Roberts 2
1 Institute of Food and Environment (York, UK)
2 Waters Corporation (Manchester, UK)
Application advantage
â– MS E data acquisition mode, which can obtain the parent ion and its fragment ion data in one injection, thus improving the credibility of compound identification. In addition, it also has the function of data traceability.
â– ChromaLynxTM XS software enables rapid detection, identification and validation of all components in complex mixtures. The user can determine the chemical formula with accurate mass information and then search and confirm the structural formula in the compound database.
â– MassFragmentTM is an intelligent software tool that automatically matches the fragment structure, greatly simplifies data processing and can be confirmed without standards.
Waters Solutions
ACQUITY UPLC ® System
ACQUITY UPLC HSS T3 Column
SYNAPT ® G2 HDMSTM System
ChromaLynx XS software
MassFragment software
Key words
Time-of-flight mass spectrometry screening, database search, structural characterization, paper, cardboard, food packaging, phthalates
Introduction
Recycling paper and paperboard can help clean the environment, reduce the pressure on forest resources, and reduce waste disposal. Currently, paper and paperboard types entering the recycling chain have certain restrictions on their use. Recycled paper and paperboard can ultimately be used in less demanding applications such as newspapers and magazines, cardboard boxes and cardboard boxes, as well as demanding applications such as food packaging. In recent years, scientific literature and the media have reported some problems with recycled paper and cardboard used in food packaging. Contaminants from recycled paper and cardboard were detected in food. Mineral hydrocarbons, 1-2 and phthalates, such as diisobutyl phthalate in adhesives used in catalogues and brochures, 3 and printed on paper and found in inks printed in newspapers and magazines Photoinitiators and other ingredients on the outer surface of the board. 4 These types of chemicals will still be present after recycling.
This research is part of a large research project that will investigate paper and board sources for recycled food packaging. 5 The experiment examined four different types of paper sources (pure white paper, newspapers and magazines, corrugated cardboard and food wrap) and identified potential contaminants. UltraPerformance LC ® (UPLC ® /HR-MS) equipped with a high-resolution mass spectrometer is an effective tool for identifying food contact materials and unknown compounds in other fields. 6 Accurate masses, isotope spectra, and fragmentation information (if any) can be used to predict elemental composition, which can then be compared to a database containing its underlying structure, and if the structure matches, the results of the identification will be more believable. The instrument used must have sufficient sensitivity and precision to ensure accurate identification of the compound.
This article describes how to use the ACQUITY UPLC/SYNAPT G2 HDMS system and related software to detect peaks, determine accurate masses, and obtain elemental components. The experiment compares the analysis results with a database of more than 6,000 food contact material components and contaminants prepared by the user, and the fragment information obtained by MS E determines one of the samples to be analyzed without using the corroborating standard. The chemical structure of the compound.
experiment
sample discription
Purchase a set of food and paper-wrapped food from a local supermarket, remove the food from the package, cut into small pieces, and mix well. Samples include breakfast cereals, pasta, frozen fish, cakes and other baked goods. A portion of the mixed sample (5 g), the internal standard d10-benzophenone (100 μL, 1 mg/mL), and ethanol (20 mL) were added to the vial, the lid was capped and shaken overnight. A portion of the supernatant was taken for analysis.
UPLC condition
System: ACQUITY UPLC
Column: ACQUITY UPLC HSS T3 (part number 176001133) 150 x 2.1 mm, 1.8 μm
Column temperature: 45 °C
Flow rate: 0.45 mL/min
Injection volume: 1 μL
Mobile phase A: water + 0.1% formic acid mobile phase B: mobile phase B: acetonitrile + 0.1% formic acid
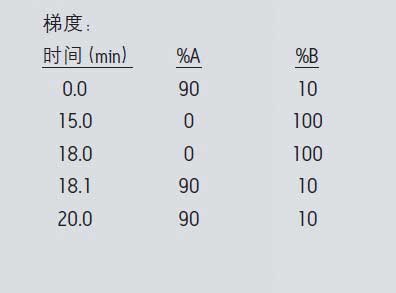
MS condition
MS system: SYNAPT G2 HDMS
Acquisition mode: MS E
Ionization mode: The mass range of electrospray positive ion detection: 50 to 1200 Da
Taper voltage: 25 V
Capillary voltage: 1.0 kV
Desolvation gas temperature: 500 °C
Source temperature: 120 °C
Collision energy: low energy CE = 6 eV,
High energy CE = 15 - 35 eV
Collision gas: argon
LockMass: Leucine Enkephalin, m/z 566.2771
Data Management: ChromaLynx XS and MassFragment Software
Results and discussion
The base peak ion chromatogram (BPI) of the ethanol extract of the mixed food packaging sample is shown in Figure 1.
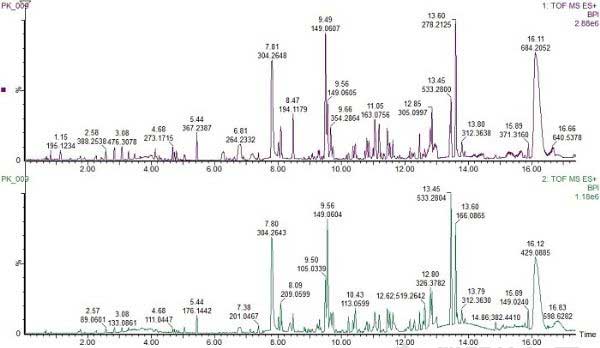
Figure 1. Base-peak ion chromatogram of ethanol extracts from paper and cardboard food packaging (low-energy electrospray ionization positive ion mode).
ChromaLynx XS software can deconvolute analytical chromatograms, detect all chromatographic components that appear, and generate accurate spectra for each identified component. These actions are performed under Target Mode and a series of individual peaks are generated, which the software then compares to the database containing the underlying structure. The software extracted 1380 components, more than what was visually observed in the TIC map. The advantages of the software for detecting components at very low concentrations are fully demonstrated. ChromaLynx XS will extract accurate mass spectra of the target compounds to determine their presence.
The user's database contains more than 6,000 known ingredients, potential contaminants, and derivatization and decomposition products that may be present in the food contact material. The list includes the compound name and chemical formula in which the software will search and report matching results. If analyzed with a standard, the retention time and fragment ion information for the compound is also included in the database. Figure 2 shows an example of ChromaLynx XS processing data, including: (A) total ion chromatogram, (B) target list, (C) extracted ion chromatogram, and (D) 13.6 min, peak correlation spectrum, which is A complete example of the qualification process. This sample was screened with a library of 6000 compounds, and a total of 45 compounds were finally identified based on the exact mass. These identification results are confirmed by the fragmentation information they simultaneously collect without the analysis of the standard.
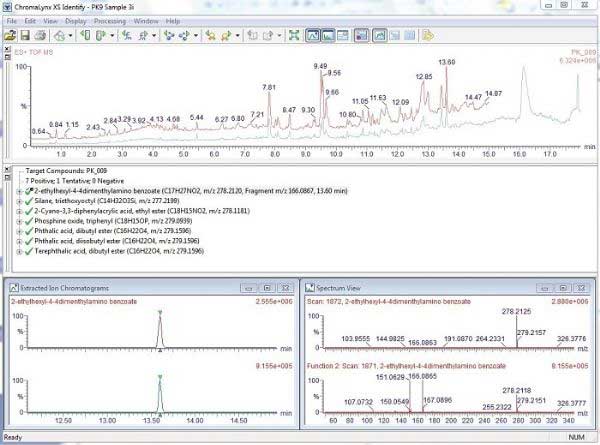
Figure 2. Mass spectrum of ChromaLynx XS output at 13.6 min, matched to isooctyl p-dimethylaminobenzoate in the database. A) Total ion chromatogram, B) Target list, C) Extracted ion chromatogram at 13.6 min (m/z 278.2122) and D) Mass spectrum (low energy) at 13.6 min.
Figure 3 shows that the parent ion has a mass-to-charge ratio of 278.2122 and a chemical formula of C17H27NO2. This is matched to isooctyl p-dimethylaminobenzoate in the database which can be used as an amine co-initiator in UV curable inks sprayed onto paper and paperboard substrates. The theoretical exact mass of the [M+H]+ parent ion is m/z 278.2120, with only a 0.7 ppm difference from the test results. In the analysis of food packaging samples, the identification of the certified standards for isooctyl dimethylaminobenzoate was not analyzed. The operating mode of SYNAPTG2 HDMS is MS E acquisition mode, which can be used for one injection and collect the parent ion and fragment ion information of the compound, thus improving the credibility of compound identification.
Figure 3 shows the low-energy and high-energy mass spectra. At higher energies, the strength of the parent ions decreases, producing fragment ions. ![Figure 3. Mass spectrum of the peak at 13.6 min. A) MS<SUP>E</SUP> high energy spectrum: showing fragment ions, B) MS<SUP>E</SUP> low energy spectrum: showing molecular adduct [M+H]+.](http://i.bosscdn.com/blog/20/13/52/813469.jpg)
Figure 3. Mass spectrum of the peak at 13.6 min. A) MS E high energy spectrum: showing fragment ions, B) MS E low energy spectrum: showing molecular adduct [M+H]+.
As with molecules, the exact mass of fragment ions can also be used to determine potential elemental composition. The MassFragment software will utilize these potential elemental compositions to confirm the structure based on the proposed chemical structure of the compound (eg, isooctyl p-dimethylaminobenzoate). The software uses systematic key break information and a set of scoring systems based on the type of bond break and the likelihood of occurrence, and the process of entering the information into the program is simple. The .mol ​​file can be downloaded from the ChemSpider commercial library or from the most commonly used chemical mapping package and then imported along with the MS E spectrum that provides fragment ion information.
According to the specific needs of the user, the parameters can be changed accordingly. The range of limits for the mass window is very important, and the smaller the range used, the higher the reliability of the structure match. In this example, the value used is +/- 1 mDa. Figure 4 is the result of the software for the peak at 13.6 min. The system recommended compound is isooctyl p-dimethylaminobenzoate.

Figure 4. Report of the MassFragment output showing the proposed structure for the five fragment ions, increasing the confidence of the identification results.
The five fragment ions tested verified the proposed parent structure—the possible structure of the ions obtained after the cleavage of different bonds in isooctyl dimethylaminobenzoate. This result improved the identification of the chromatographic peak at 13.6 min. Reliability. Figure 5 shows an MS E mass spectrum labeled with a MassFragment structure. This compound is likely to come from inks on paper and paperboard, and 7 similar chemical types of compounds will still be present after recycling. Now, both the fragment ions and the retention time match this compound, and they are fed back into the database, making subsequent identification more credible.
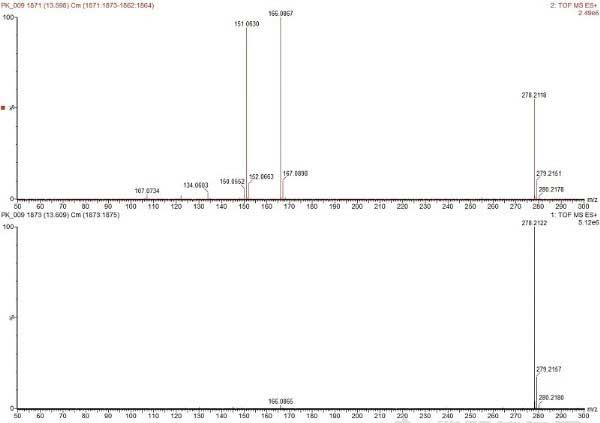
Figure 5. MS E mass spectrum of the peak at 13.6 min, labeled with MassFragment.
in conclusion
In this experiment, the ACQUITY UPLC/SYNAPT G2 HDMS system with chromatographic separation and high-resolution determination of accurate mass is used to analyze food packaging paper and paperboard extracts. This analysis allows for the trustworthy identification of previously unknown compounds that may migrate into food. Using MS E data acquisition mode, the information of the parent ion and the fragment ion can be collected at the same time, and the collected data can be processed by ChromaLynx XS and MassFragment software to obtain high-confidence identification results.
references
1. Dima G, Verzera A, Grob K. Migration of mineral oil from party plates of recycled paperboard into foods: 1. Is recycled paperboard fit for the purpose? 2. Adequate testing procedure. Food Additives and Contaminants Part A.2011; (11): 1619-1628.
2. Vollmer A, Biedermann M, Grundbock F, Ingenhoff JE, Biedermann-Brem S, Altkofer W, Grob K. European Food Research and Technology. 2011; 232:175- 182.
3. Gartner S, Balski M, Koc h M, Nehls I. Analysis and migration of phthalates in infant food packed in recycled paperboard. Journal of Agricultural and Food Chemistry. 2009; 57(22): 10675-10681.
4. Koivikko R, Pastorelli S, deQuiros ARB, Paseiro-Cerrato R, Paseiro-Losada P, Simoneau C. Food Additives and Contaminants Part A. 2010; 27(10): 1478- 1486.
5. Driffield M, Lloyd AS, Lister L, Leak J, Speck D, Bradley EL. Manuscript in preparation. 2013.
6. Driffield M, Bradley EL, Castle L, Coulier L. Identification of unknown migrants from food contact materials. Mass Spectrometry in Food Safety, Methods and Protocols. 2011; 357-372.
7. Food Standards Agency (2011) Food Survey Information Sheet 03/11. Migration of selected ink components from printed packaging materials into foodstuffs and screening of printed packaging for the presence of mineral oils.
Capillary Microcirculation Microscope
Micirculation Microscope:
The Nailfold Capillaroscopy Microcirculation Microscope is an advance medical photoelectric apparatus,equipped with built in special LED light source,used mainly in observation on human nail fold capillary microcirculation or term as video Nailfold capillaroscopy,Such as capillary blood flow, abnormal microcirculation of the vascular structure, cell adhesion, through its powerful optical magnification. Undistorted, real time dynamic video streaming via Sony CCD imaging device onto the LCD monitor screen.
Digital Microscope,Micirculation Microscope,Blood Microcirculation Microscope,Microcirculation Microscope Device
Shenzhen Guangyang Zhongkang Technology Co., Ltd. , https://www.szlighttherapymachine.com