In recent years, the basic research in the medical field has become more systematic and multidisciplinary, and the rapid development of system biology has opened a new chapter in clinical practice and drug research and development. As a research team of Professor Jia Wei who was involved in system biology research earlier in China, in recent years, he has used metabolomics technology to conduct extensive research on biomarkers for early prediction, diagnosis and prognosis of various clinical diseases. Now he is a colorectal cancer. The series of studies is an example to introduce our research progress.
The research team first used gas chromatography-mass spectrometry, liquid chromatography-mass spectrometry, combined with single-dimensional statistics, multidimensional statistical metabolomics research techniques, 64 patients with stage I-IV and 65 healthy volunteers. Serum and urine metabolic markers were screened separately, and the potential metabolic markers found were further validated in 101 enrolled cancer patients and 103 healthy individuals.
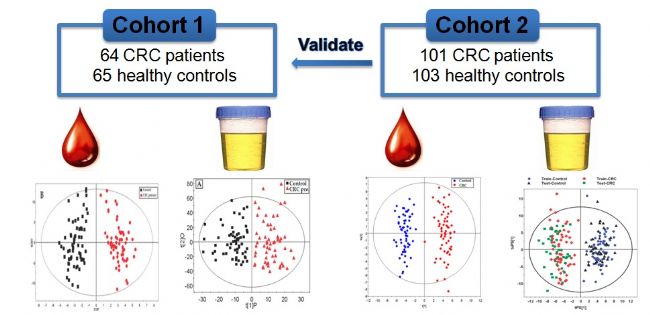
The results of the study showed that there was a significant difference in the composition of serum metabolites between patients with intestinal cancer and healthy people. The two metabolites pyruvate and lactic acid in the glycolysis pathway of patients with intestinal cancer showed a significant increase in serum, and the succinic acid, isocitric acid and citric acid intermediates in the tricarboxylic acid cycle showed a downward trend; There was also a significant decrease in the serum levels of patients with intestinal cancer; the urea cycle metabolites arginine, ornithine and citrulline were significantly reduced in the serum of patients, and proline, hydroxyproline and glutamic acid were also significant. In addition, tryptophan and its related metabolites 5-hydroxytryptophan and 5-hydroxyindoleacetic acid were significantly different between the intestinal cancer group and the normal group, suggesting a correlation with the metabolism of serotonin. The results also show that serum metabolites can not only clearly distinguish patients with stage II-IV of bowel cancer from healthy people, but also distinguish patients with stage I early stage cancer from healthy people. Our relevant research results have been published in the Journal of Proteome Research (2009 and 2013), which has been influential in the professional field since 2009.
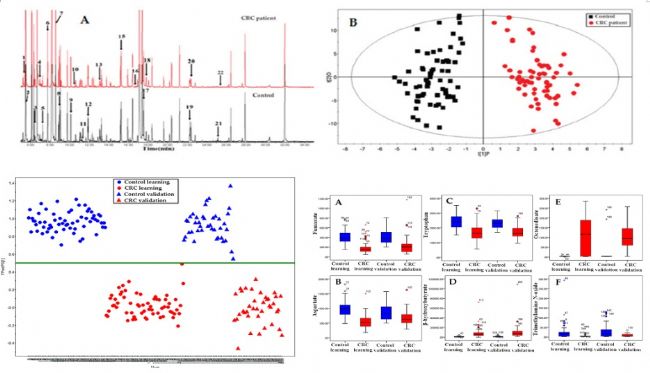
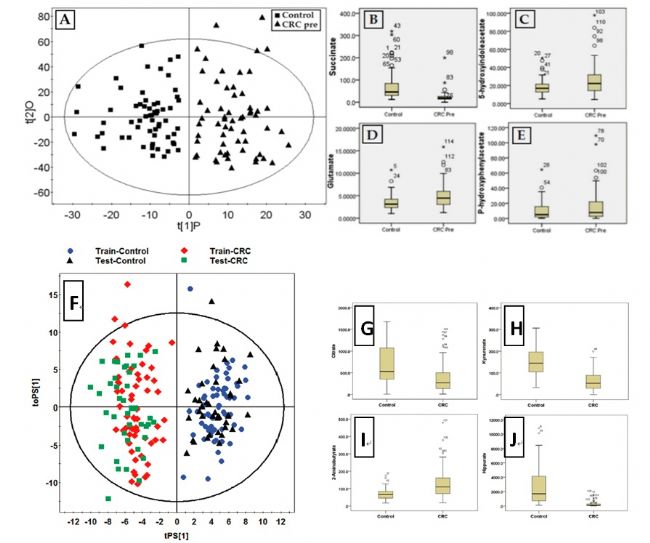
The results of urinary metabolomics also showed significant differences in the metabolic profiles of colorectal cancer patients and normal subjects. Tryptophan metabolism is upregulated in colorectal cancer patients, histamine and glutamate metabolic pathways, tricarboxylic acid cycle, and intestinal flora metabolic disorders. In addition, metabolic profiles of disorders in colorectal cancer patients, such as 5-hydroxytryptophan metabolites, tricarboxylic acid cycle metabolism, and intestinal flora metabolites, are significantly improved after surgery. The study further carried out a study on the SD rat model of early stage of colon cancer caused by dimethylhydrazine (DMH), and also found fluctuations and disorders of these metabolites. The results of the study were published in the Journal of Proteome Research (2010 and 2012), and received key reports and attention from a number of authoritative media represented by ACS and TIME (Times of the Week). The results and prospects of the study were highly evaluated. .
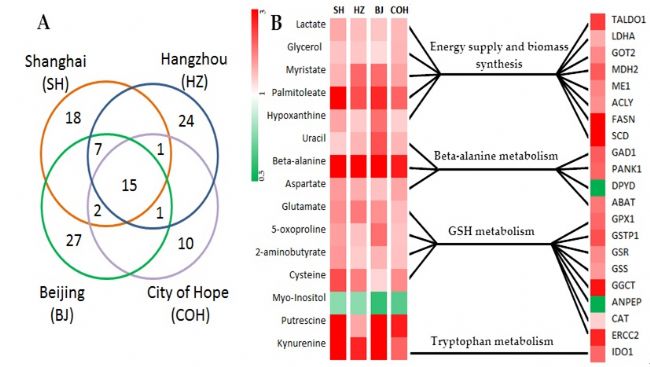
Based on the metabolomics study of serum and urine of colorectal cancer, we have also conducted in-depth research on the organization of intestinal cancer. The research on tissue can effectively avoid the external factors such as dietary differences in serum and urine research. The impact of the material has an impact on the results of the study. The team first studied colorectal cancer and paracancerous tissues from Shanghai and found a group of metabolites with significant differences in cancer and adjacent tissues. Further, colorectal cancer and paracancerous tissues from three different regions of Beijing, Zhejiang, and California were also studied. The results showed that the trend of total metabolites in intestinal cancer tissues was highly similar in four different regions, and 15 of them showed a completely consistent trend. Further studies have found that these differential metabolite changes are highly consistent with changes in gene expression levels in the metabolic pathways in which they are located. These differential metabolites include up-regulated kynurenine, -alanine, glutamic acid, cysteine, 2-aminobutyric acid, palmitoleic acid, pyroglutamic acid, aspartic acid, hypoxanthine, Lactic acid, myristic acid, glycerin, uracil, putrescine, and down-regulated inositol. Differentially expressed genes include LDHA, TALDO1, GOT2, MDH2, ME1, GAD1, ABAT, PANK1, DPYD, ACLY, FASN, SCD, IDO1, GPX1, GSTP1, GSR, GSS, GGCT, ANPEP, CAT, ERCC2. Metabolite patterns and gene expression patterns of colorectal cancer found in combination with metabolite and gene expression changes can be explained in three aspects: 1) Warburg Effect: This is the tumor cell energy A typical characteristic of metabolism is the large intake of glucose for aerobic glycolysis, the production of large amounts of lactic acid, while providing biosynthetic raw materials for growing tumor cells; 2) for the growth of glycolysis, for macromolecular substances Synthetic metabolic intermediates are significantly elevated: the metabolism of tumor cells produces macromolecular intermediates to support cell growth, leading to an increase in the concentration of certain specific free fatty acids (mycophenolic acid, palmitoleic acid) and nucleic acids (hypoxanthine). In tumor cells, high expression of ACLY, FASN and SCD also suggest an increase in fatty acid synthesis. However, the obvious changes of alanine in tumor cell growth may be closely related to acetyl-CoA and malonate-CoA in fatty acid synthesis, suggesting that this change may be related to intestinal flora metabolism; 3) Maintaining high levels of oxidative stress in tumor cells: We found a significant increase in the concentration of antioxidant active metabolites in tumor tissues. As the tumor cells accelerate anabolism to produce higher reactive oxygen species, the level of intracellular oxidative stress increases. These metabolites with antioxidant activity have been synthesized in large amounts in tumor tissues, suggesting that tumor cells maintain their metabolism at a higher level of oxidative stress by changing metabolic patterns and balancing reactive oxygen species with reducing molecules. Physiological and metabolic functions. It was found in the experiment that the biomarker of oxidative stress, as the crystalline acid and 2-aminobutyric acid, rose in tumor cells. At the same time, glutathione-related genes including GPX1, GSR, GGCT, and GSTP1 were also significantly elevated in tumor tissues. The findings were published in the internationally renowned journal Cancer Research (Clinical Cancer Research (2014)).
We believe that systematic metabolic studies of colorectal cancer offer great potential for the search and discovery of biomarkers with early clinical diagnosis and prognostic value, and lay a solid foundation for future clinical transformation studies.
Original source:
1. Qiu, Y.; Cai, G.; Su, M.; Chen, T.; Zheng, X.; Xu, Y.; Ni, Y.; Zhao, A.; Xu, LX; Cai, S.; Jia , W., Serum metabolite profiling of human colorectal cancer using GC-TOFMS and UPLC-QTOFMS.Journal of Proteome Research. 2009, 8, 4844–4850.
2. Qiu, Y.; Cai, G; Su, M.; Chen, T.; Liu, Y.; Xu, Y.; Ni, Y.; Zhao, A.; Cai, S.; Xu, LX; W., Urinary Metabonomic Study on Colorectal Cancer. Journal of Proteome Research. 2010, 9, 1627–1634.
3. Cheng, Y., Xie, G., Chen, T., Qiu, Y., Zou, X., Zheng, M., Tan, B., Feng, B., Dong, T., He, P., Zhao, L., Zhao, A., Xu, LX., Zhan, g Y., Jia, W. Distinct urinary metabolic profile of human colorectal cancer. Journal of Proteome Research. 2012, 11(2): 1354-63.
4. Tan, B, Qiu, Y, Zou, X, Chen, T, Xie, G, Cheng, Y, Dong, T, Zhao, L, Feng, B, Hu, X, Xu, L. X, Zhao, A, Zhang, M, Cai, G, Cai, S, Zhou, Z, Zheng, M, Zhang, Y & Jia, W. Metabonomics, marker serum metabolite markers of colorectal cancer. Journal of Proteome Research 2013, 12, 1354?1363.
5. Qiu, Y.; Cai, G.; Zhou, B.; Li, D.; Zhao, A.; Xie, G.; Li, H.; Cai, S.; Xie, D.; Huang, C.; Ge, W., Zhou, Z.; Xu, L.; Jia, Weiping; Zheng, S.; Yen, Y.; Jia, W. Metabonomics of human colorectal cancer: new approaches for early diagnosis and biomarker discovery. Research.2014, 20(8): 15.
Antacids,Anti Acid Medication,Best Antacid,Antacid Medicine,Antacid Products
NOUVASANT GROUP LTD. , https://www.nouvasant.com