Release date: 2016-11-02

[Health Point] In recent years, with the increasing living standards and unfamiliar eating habits, the incidence of diabetes is getting higher and higher. According to a study by the Centers for Disease Control and Prevention (CDC), about 40% of American adults have diabetes (mainly type 2 diabetes) in their lifetime, and some ethnic minorities may even have a prevalence of more than 50%.
In the moment, medical and big data are a hot topic that continues to be a concern. The Obama Administration’s Cancer Mortal Plan and Precision Medical Initiative have made Big Data the goal of work. Diabetes, as a common chronic disease, is much more dependent on the management of “data†(including big data and small data); the control of diabetes is also largely determined by the management of blood sugar levels, so it is very suitable Develop a variety of artificial intelligence (AI) and applications.
In the past few years, data-driven diabetes management has been in full swing: Dynamic Blood Glucose Monitoring (CGM) technology continues to improve, and some companies that seem to be out of bounds have established partnerships, the application of automated insulin delivery systems (artificial pancreas). It is getting closer and closer to us.
Artificial pancreas
In the artificial pancreas, Medtronic, the world's leading medical technology company, took the first step on this long road: its hybrid closed-loop automatic insulin delivery system MiniMed 670G became the first FDA-approved device. The system uses a new algorithm, SmartGuard, to provide a dose of insulin for patients with type 1 diabetes.
(Medtronic's MiniMed 670G)
However, artificial pancreas is not a panacea. Even the newly approved Medtronic MiniMed 670G still requires people to manually enter carbohydrate intake, calibrate the device every 12 hours, adjust the blood glucose sensor once a week, and add a drug to the insulin reservoir every 3 days...but Compared with the traditional hourly blood test method, Medtronic has made great progress in blood glucose control technology.
In addition, Medtronic collaborated with IBM's Watson Medical to create a cognitive application that uses Medtronic's insulin pump and big data to predict patient blood glucose trends. The tool is dedicated to providing users with better glycemic control methods that alert patients 3 hours before the onset of hypoglycemia.
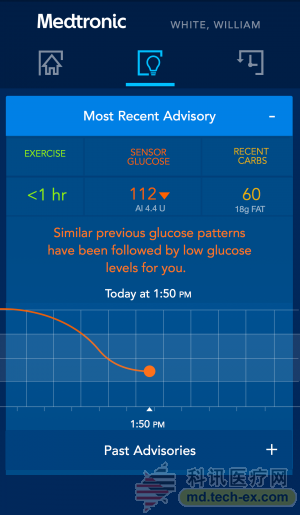
(Maylorson-Watson warning tool interface screenshot)
In addition to Medtronic-Watson, Glooko, a digital healthcare company focused on pre-diabetes management, has developed similar early warning technologies. The company has been working with the Joslin Diabetes Center in Boston to provide timely injections of glucagon in patients with type 1 diabetes through a hypoglycemic early warning technique. Glooko aggregates patient data from wearable devices and applications to gather information about meals, exercise, and sleep.
Dynamic blood glucose monitoring
At present, the products of dynamic blood glucose monitoring mainly form an electrical signal through the sensor that penetrates into the skin, and the patient's interstitial fluid reacts with the glucose in the body, and then converts into a blood glucose reading, which is transmitted to the wireless receiver through the transmitter.
In this area, Dekang Medical, a manufacturer of dynamic blood glucose monitoring systems based in San Diego, California, is at the forefront of the world. In early 2015, Dekang received an FDA license for the mobile app "Dexcom Share System" bundled with its G4 Platinum CGM. The application is divided into two versions, the administrator and the patient. The former can connect Dekon's devices to up to 5 designated managers or family members' smartphones, while the latter can be connected to the patient's smartphone. on. This "attention" function is especially important for type 1 diabetes, which helps relatives to understand the patient's condition at all times to avoid complications such as hypoglycemia or hyperglycemia.
Daniel McCaffrey, director of digital health, data and analysis at Dekang, is proud to say that CGM is a huge advancement in management, management and data. It more accurately describes the patient's health and diabetes control status, and it is easier and more convenient to collect data than previous self-monitoring blood glucose (SMBG).
Type 1 diabetes patients perform multiple finger blood glucose tests every day, and CGM devices can read readings every 5 minutes. Users only need to measure blood glucose 2 to 3 times a day to calibrate the instrument. Moreover, CGM not only has time and data advantages for patients with type 1 diabetes, but also has similar effects for patients with type 2 diabetes.
When the CGM system is connected to other devices and bound to management algorithms, it will become even more powerful. For this reason, although Dekang's G4 series monitors have been on the market for several years, the FDA's approval of its application Dexcom Share System has really made it epoch-making. Because of it, the G4 can be connected to other devices.
In this ecosystem created by Dekang, people can design a variety of products that support G4. Its CGM instruments can now be paired with insulin pumps, clinician dashboards, and various third-party patient nutrition and activity trackers. McCaffrey said that Dekon will soon release the programming interface for the application, and then its ecosystem will grow geometrically.
Type 2 diabetes tool
While companies such as Medtronic and Dekang are working to improve the lives of people with type 1 diabetes, Omada Health, a San Francisco-based digital healthcare company, is focused on helping patients with pre-diabetes.
Omada believes that weight is important for diabetes control because weight gain is one of the signs of increased risk of diabetes. And the acquisition of weight data is much easier than hemoglobin. In addition, Omada also values ​​the interconnectivity of the product: it has a built-in 3G connection to the scales of its remote diabetes management program, eliminating the need to work under a Wi-Fi network or pair with a mobile phone or tablet via Bluetooth. It is very convenient to use.
Of course, in the prevention and treatment of type 2 diabetes, it is far from enough to monitor body weight. People often need other supplementary data. But in today's big data trend, medical staff often do not lack data, but the organic integration of data. Omada's role is to help users understand data such as scales, fitness trackers, patient-reported food intake, and even information on group message boards, and integrate these data into lifestyle recommendations and clinical decisions. Not complicating treatment.
According to Eric Williams, director of data science at Omada, Omada's algorithm can alert users to the risk or symptoms of diabetes through changes in body weight. In the future, Omada will provide better interventions and treatments through continuous data tracking and improved algorithms.
This algorithm is not perfect at the moment. For example, Omada initially issued a pedometer to each participant and set a goal of “10,000 steps†every day, but different participants gave different feedbacks, some thought the goal was too difficult, and some Also think too easy. This made Omada quickly realize that the demographic composition of the participants would affect the experimental results. As a result, Omada immediately grouped participants by indicators such as body mass index, age, and gender, and developed more personalized goals for each group.
Personalization and prediction are the general direction of diabetes management development. To date, Omada has recruited 75,000 volunteers to participate in the Diabetes Prevention Program, which has been weighted by 10.5 million times, generated more than 1 billion data points, and has the largest data set on behavioral changes. Now, for a particular patient, Omada can predict the behavior of the individual after 16 weeks with an accuracy of 80% for three to four weeks.
In general, data-driven diabetes prevention and treatment have achieved some results, but people still have a lot of work to do, especially the potential of data in diabetes management needs further development.
Source: Health Point
There are two types of dietary fibre - soluble and insoluble - and sweet corn contains both.
According to the American Heart Association, dietary fibre as part of an overall healthy diet helps to lower blood cholesterol levels and may reduce the risk of heart disease. It is insoluble fibre that binds to cholesterol, preventing it from being absorbed into the bloodstream.
Insoluble fibre is responsible for promoting regularity and helping to prevent constipation by speeding up the passage of food and waste through the intestines and absorbing water to keep stools soft. Insoluble fibre has been shown to reduce the risk of haemorrhoids.
Fibre-containing foods such as sweet corn also help to provide a feeling of satiety and may therefore help to suppress appetite and aid weight management.
Dietary fibre has also been linked to a reduced risk of type 2 diabetes. A diet rich in fibre helps patients manage their disease.
Fibre is fermented by bacteria in the colon. Promising studies are underway to determine the health-promoting effects of fibre fermentation breakdown products, for example, short-chain fatty acids, which may help to maintain a healthy gut.
Non Gmo Hominy Corn,Butter Maize,Mini Sweet Corn Kernels,Sweet Cream Corn
Jilin Province Argricultural Sister-in-law Food Co., Ltd. , https://www.nscorn.com