Release date: 2016-08-18

Rock Health is an incubator similar to Y Combinator, located in San Francisco, USA, providing entrepreneurial teams with office space, industry instructor guidance, startup funding and more. Rock Health recently published a report on genomics. The arterial network has been translated and reorganized, and it is expected to provide some inspiration for the increasingly competitive genomics application companies.
Genomics has enormous potential and will have a major impact on the healthcare industry. This is due to a large number of high-profile corporate and individual investments and initiatives, such as President Obama's proposal for precision medicine (1 million people joined this month), and consumers are beginning to pay more attention to genomics and its position in the medical industry. .
The genetic code is currently under development in the medical industry, and doctors, scientists and technicians in this field have a long way to go. In the course of writing this report, we found that genomics is at an important turning point. Despite the breakthrough in gene sequencing technology, the cost of sequencing is also significantly reduced, but gene sequencing is not very attractive to the public, and it is not closely integrated with medical institutions such as hospitals.
The development prospects of genomics depend mainly on three factors, mostly related to digital medicine:
(1) Ensure the diversity of sources of data sets that provide insights
(2) Breaking down barriers to cooperation with medical institutions
(3) Promote scientific and technological progress in the laboratory and the cloud
To understand the potential methods and current barriers to the promotion of genomics, we interviewed a thousand consumers, provided new data and insights about the willingness to purchase specific use cases, and explored privacy and ownership issues.
There is no doubt that genomics will improve the medical industry and promote personalized medicine. But the science of genomics has been esoteric for decades. Therefore, it is equally difficult to extend genomics to the medical care industry. We hope to provide some inspiration for the promotion of genomics and technological advancement through research.
Research Background
Sajith Wickramasekara, founder and CEO of Benchling, mentioned a question. Is it more appropriate to say that genetic data is more objective than other types of data? Will all data become binary result data? Although there are still a lot of unsolved mysteries, if we have tried other methods, this may be the final answer to those problems.
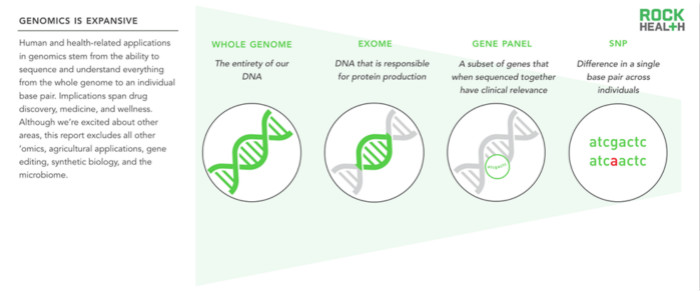
The influence of the field of genomics is enormous. This report examines the application of all medically relevant genetic data, including single nucleotide polymorphisms (SNPs), gene panels (a group of genes that are clinically meaningful after sequencing), and exomes (1-2% of genomes). Forming a protein) and a whole genome containing three million base pairs. Other biogroups (such as proteomics, metabolomics) are outside the scope of this report.
We use the terms “genomics†and “gene detection†in our report to distinguish the differences between terms: genomics is the study of large segments or genome-wide variations, while genetic testing involves only specific genes.
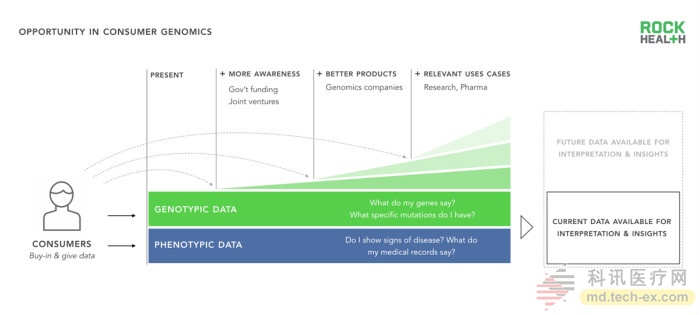
One of the primary goals of genomics is to provide personalized, actionable solutions to provide better care. Achieving such goals still requires understanding how genes and human health interact, and scientists need more diverse genetic and phenotypic data (such as personal information) and free access to relevant data sources.
When consumers buy direct-to-consumer (DTC) genomic products, such as choosing to go to the doctor's office to do genetic data in the genetic testing ecosystem, or participate in clinical tracking studies, genetic data in the ecosystem will increase. The genomic industry can and should encourage consumer engagement at these touch points. At present, this is mainly achieved through three methods: (1) understanding of the field of genomics (2) providing consumers with better genomic products (3) more relevant user use cases.
The US government has increased public awareness of genomics by funding grants for some of the most well-known proposals, such as precision medical proposals. Business companies have also made contributions, such as 23andMe, which is committed to providing products that are easier to access and easier to understand. Finally, diagnostic companies and research institutes such as The Broad Institution are planning new collaborative projects and using user stories to generate customer interest in personally oriented products.
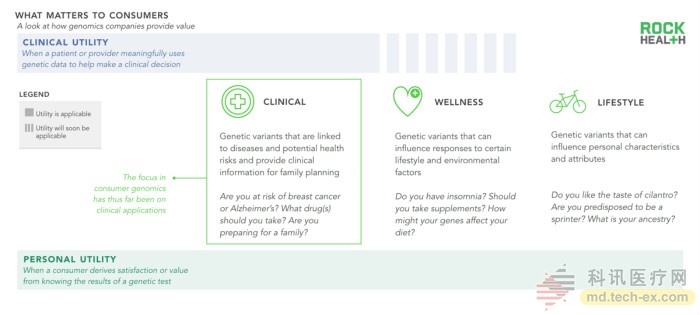
Through genetic testing, consumers or patients can benefit (or gain utility) in two ways – clinical utility and personal utility. Clinical utility is relatively clear: Is genetic testing likely to change my treatment course? Is it possible to change my decision with a doctor to know if I belong to a high-risk group of certain diseases? Personal utility is more subtle, mostly depending on how the consumer processes and uses the genetic information. For example, do you know if my family tree will bring happiness? Does it make sense to share this genetic information to family members or doctors, even if the contribution of this information to clinical treatment is minimal?
We divide the user stories of genomics into three categories: clinical, health, and lifestyle. Clinical genetic testing helps patients understand whether they are at high risk for certain diseases. Special genotypes (usually oncology drugs) based on the patient's disease can also be applied to the birth plan. Clinical testing includes most of the currently available genetic tests. Health genetic testing can provide information about chronic diseases, diet, and exercise, but it does not currently provide valuable clinical information. The genetic test of lifestyle can satisfy consumers' curiosity about themselves and has no direct connection with medical health.
Although we have carefully studied the application of genomics directly to consumers, it is understood that nearly three-quarters of genomics companies provide tools (both physical and cloud) for pharmaceutical companies and academic institutions. Scientists and researchers use these tools to speed up drug development and develop more personalized medicines – represented by oncology-related applications.

Although most genomics companies currently only conduct research in the life sciences, this is gradually changing as technology forces extend beyond research laboratories. For example, there are nearly 40 venture capital genomics companies in the health care ecosystem, many of which aim to create a more personalized ecosystem. For example, Syapse Corporation achieves these goals by providing integrated tools for clinical workflows; other companies, such as InformedDNA, provide point-to-point genetic testing solutions that omit patient counseling processes. Other companies offer diagnostic protocols, most of which are related to prenatal testing and oncology drugs, and are sold to doctors who connect patients and testing services. Only a few companies provide genetic testing services directly to patients, mainly in the family lineage field, which poses challenges for the regulation of direct-to-consumer markets.
Very few companies provide services directly to payers and employers, and promise customers that their products can reduce the cost of health care services through advanced predictive technology. For example, BaseHealth provides a platform for payers and employers to understand the risk factors of the public's disease and use genetic data as a reference for reducing the risk of illness. These tools are not yet mature, perhaps because of the lack of the basis and motivation to carry out systematic reforms.
Business model
Many genomics business models are composed of data. In the field of genomics, as in the health care arena, data ownership (when and how to handle data control) is often in the gray of the law. Although the Obama administration has issued a bill on patients' access to case information (including genetic test results), stakeholders who “own†genetic data are not necessarily entities that analyze and understand the data.
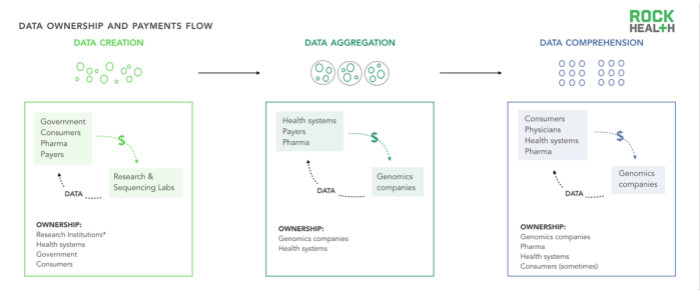
As an example, we assume that the healthcare system hopes to better understand the impact of oncology applications on specific populations. In order to achieve this goal, the system will pay for a third-party vendor to collate and compile a variety of data sources, and rely on doctors to invite patients to do genetic testing or research. The role of these three stakeholders is critical in the data flow; however, there is no clear definition of who owns the ownership of the sample, identified data, and unidentified data. Is it a health care system? Data collector? Or the consumer himself? In most cases, this depends on the details of the study and the patient consent form. In addition, DNA and data itself come in many forms and can be stored in the cloud of gene banks or healthcare system servers.
Policies to clarify ownership remain to be improved, not only to protect consumer privacy, but also to companies that extract value from consumer data as a business model. Companies entering the field of genomics with data collection strategies need to be prepared to mobilize the complexity of data and funding, and to adapt to the rules that may change.
The business model of many genomics companies relies on exclusive customer data (phenotypic and genetic). In the future, two business models of data ownership may emerge. Currently, most consumers choose only one genomics company and only provide one genetic sample. However, as the cost of genetic testing has decreased, companies are expanding their businesses and providing differentiated services. Consumers are likely to provide genetic samples or processed DNA to multiple companies. Hliex is preparing to launch a third business model: Consumers only provide one genetic sample but can use user stories indefinitely.
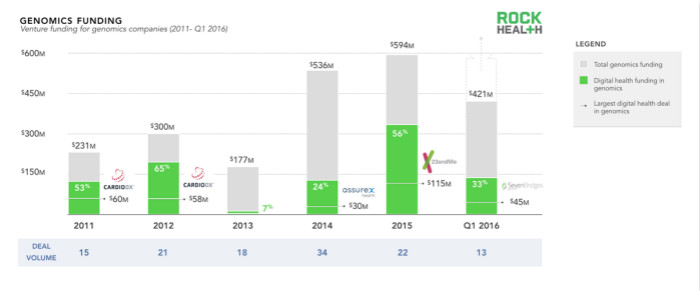
The genomics industry brings together a large number of public and private investors, which also proves the potential of genomics. We have observed an increase in the number of venture capital investments in the past five years, with a total investment in genomics companies exceeding $2.2 billion. The growth from the first quarter of 2015 to the first quarter of 2016 was attributed to several major transactions. In 2015, Helix and 23andMe both received more than $100 million in investments; in the first quarter of 2016, Grail and Guardant also received $100 million in investments.
Although genomics has always been strongly associated with life sciences, it has only recently been combined with technology and cloud solutions on a large scale. In the last three years, digital medical genomics companies (genomics companies with the necessary technology components) have invested in half of the total investment of genomics companies. With easier-to-understand products, more relevant use cases, and better integration of clinical workflows, the development of science and technology continues to receive widespread attention from consumers, healthcare organizations, and healthcare systems. As the technological advances in life sciences make research more efficient, digital healthcare is expected to apply genomics to healthcare.
Due to the large number of clinical trials and investment events (such as Guardant and Grail), recent liquid biopsy techniques (for the detection of non-invasive cancers) have caught our attention. Although the actual clinical application is still in the early stages of development, this technology will be applied and promoted when good test results are obtained.
Finally, although we have not delved into the investors behind genomics, it is worth mentioning that Illumina has been driving the industry's development and progress. In addition to being an industry leader, Illumina also split two potential transformation companies (such as Grail and Helix) to invest in a group of early start-ups through the Enterprise Acceleration Program and recently announced a $100 million venture capital investment. Funds (Illumina Venture) are used to plan to invest in more startups.
To better understand how companies package tools and services into products, we analyzed five elements of the genomics value chain: sequencing, analysis, interpretation, aggregation, and market. Currently, nearly 40% of venture capital genomics companies are able to provide professional solutions and focus on only one element of the genomics value chain, without companies providing services across the entire value chain. One reason is the highly specific needs of researchers and laboratories, and many suppliers choose to use specific user cases to serve only a small number of customers.
The demand for fully outsourced diagnostic solutions is high, with a large number of genomics companies (39% of venture capital firms) offering accompanying sample-to-insight products to healthcare facilities and healthcare systems. Most companies that offer gene sequencing offer "sequencing-as-a-service" services and apply Illumina's Next Generation Sequencing (NGS) technology to the service process itself. However, no company's services exceed 10% of the sample, which indicates that other companies' business models cannot obtain value or are not feasible.
A new element has recently emerged in the value chain: the market. The market is a central repository of data that provides a channel for all stakeholders to access data. The presence of the market allows consumers to provide only one sample, and as the company launches new user stories and related research, consumers can continue to derive value and service from the sample. Helix, funded by Illumina and other investment companies, is the only genomics company to receive venture capital and market-based products.
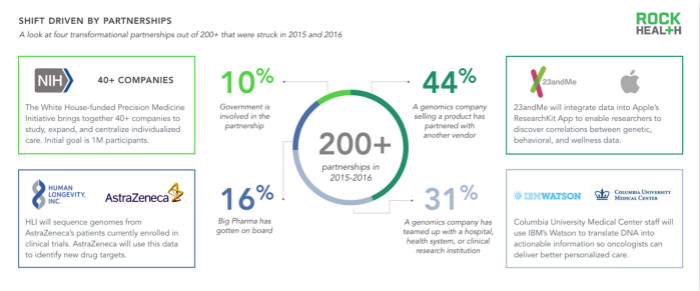
Most companies do not offer end-to-end solutions, and the development of genomics companies is often driven by partners. To better understand the drivers between such companies, we created a database of 200 corporate partnership projects. (January 2015 to April 2016)
The largest category in the database – accounting for 50% of the collaborative projects analyzed – is a collaborative project for the product. For example, in March 2016, 23andMe announced that its software will be integrated with Apple's ResearchKit App to allow customers to share genetic data with researchers. With the customer's consent, the researcher can analyze the customer's behavior/phenotype data (collected via the ResearchKit App on the iPhone or Apple Watch).
Collaborative projects between government agencies and private companies (10% of the database) are strongly promoted by Obama's Precision Medical Proposal (2015). The initial goal was to focus on the development of cancer research, which will then be extended to research on common diseases such as diabetes and Alzheimer's disease. The White House has approved funding for the National Institutes of Health (NIH) $130 million, the National Cancer Institute (NCI) $70 million, and the Food and Drug Administration (FDA) $10 million, National Center for Health Information Technology (ONC) 5 million US dollars. For example, the FDA's collaborative DNA project with the DNA cloud database startup DNAnexus will create a service platform that integrates genetic testing results and ensures high quality standards.
Pharmaceutical companies and genomics companies have begun to jointly explore new therapies associated with genotypes and phenotypes. One of the largest collaborative projects is the one-year contract between HLI and AstraZeneca announced in April 2016: HLI will sequence the 500,000 genomes of AstraZeneca clinical trial participants. AstraZeneca plans to apply data insights to drug development goals and enrich HLI's knowledge base, while HLI claims to have the most comprehensive knowledge base in the industry. At the same time, AstraZeneca can also conduct clinical trials through HLI-specific knowledge base, strengthen biomarker research, and promote the development of drug development.
Finally, we have compiled a case of nearly 60 medical systems and suppliers working together to provide precision medical services. Syapse's partnership with Intermountain Healthcare and IBM's Watson and Columbia University Medical Center are the best examples of two medical systems that derive valuable information from genetic data to develop personalized treatment options for cancer.
Consumer sentiment
Consumer adoption of genetic testing services is key to researchers gaining insight and value from genomics because there is more information for researchers to use and analyze. While stakeholders have made some predictions about consumer preferences for genetic data, we hope to provide new data on consumers' willingness to choose and purchase specific use cases, and overall trust in the company's ability to inform new products and services.
We surveyed more than a thousand representative adults and tried to ask them to answer the following questions: (1) How does the use of genetic testing vary from person to person? (2) What are the people's attitudes toward data sharing, ownership and privacy? (3) Does the public think that genetic data is different from traditional medical data? (4) How much do people are willing to pay for their health information?
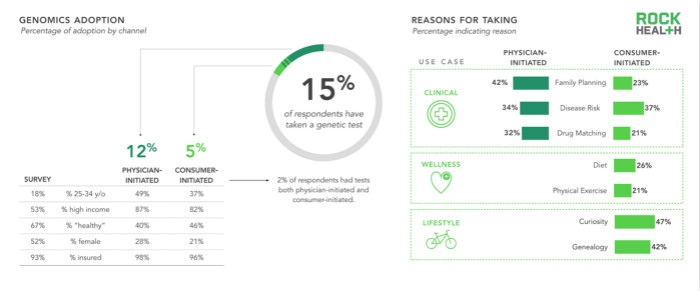
n = 1,060
The study found that only 5% of the samples will actively purchase genetic testing services, and 12% of the samples will follow the doctor's advice for genetic testing. As predicted, people actively purchase genetic testing services for health and lifestyle reasons, related to diet, exercise, curiosity and genealogy. Most users want information about birth plans, disease risks, or responses to a drug's genotype.
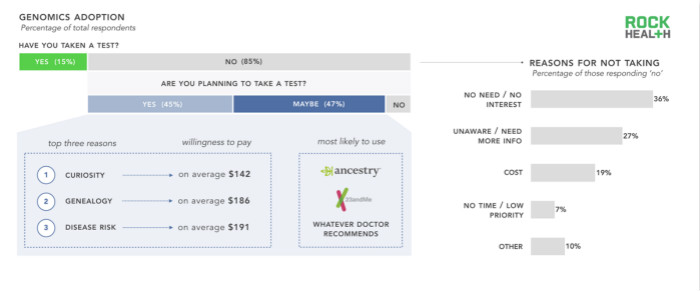
n = 1,060
Most of the survey samples and American adults have not yet received genetic testing, and we are curious about what is behind them. In addition, we also want to know what some people plan to accept genetic testing in the future. The most common answer to our open question (why did not receive genetic testing) is that there is no need to do testing, and then there is no need to know more about the available tests and decisions before testing. Only one in five responded to genetic tests because of cost issues.
Although many respondents who have done genetic testing are testing for health reasons, the top three reasons for planning for genetic testing in the future are related to curiosity, genealogy, and disease risk. Although a large number of startups tested for health intentions, most respondents did not include health as a reason for receiving genetic testing. Ancestry.com and 23andMe are the genomics companies selected by most consumer programs, and most respondents will follow the doctor's advice, whether it is about clinical, health or lifestyle.
n= 1,060
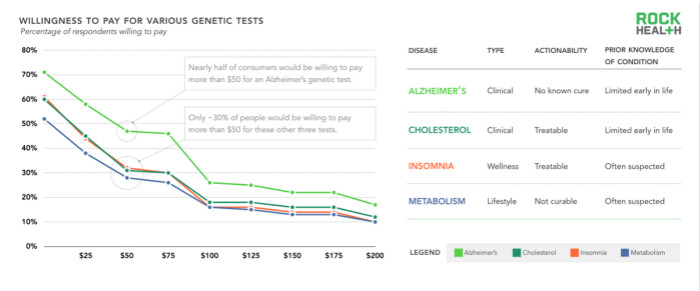
Given the concerns of direct-to-consumer testing and the application of genetic testing in clinical settings, we hope to judge consumer acceptance of various state-based tests from the following dimensions: (1) User stories (clinical, health, and/or Lifestyle) (2) operability level (whether a genetic test can lead to a specific clinical intervention effect?) (3) prior knowledge about the known state (eg, the user's understanding of the situation before receiving the genetic test) . We asked respondents how much they were willing to pay for genetic testing. This genetic test predicts whether patients will develop four diseases: Alzheimer's, high cholesterol, insomnia, and slow metabolism.
We found that consumers are more willing to pay for genetic testing for Alzheimer's disease than genetic testing for high cholesterol, insomnia, and slow metabolism. For example, 47% of consumers are willing to pay $50 for detecting Alzheimer's disease, while only 28% are willing to spend the same amount of money to detect metabolism. In addition to family history, it is difficult to know the odds of getting Alzheimer's disease, but genetic testing provides new clues for the future. In contrast, people are least willing to pay for genetic testing that is slow to metabolize, probably because the test does not provide information that is currently unknown.
Finally, it is worth mentioning that we have found that respondents are willing to pay the same question whether they are positively (recognized to have a genetic test for a certain disease) or negatively (confirm that genetic testing for a disease will not be obtained). of.
n= 1,060
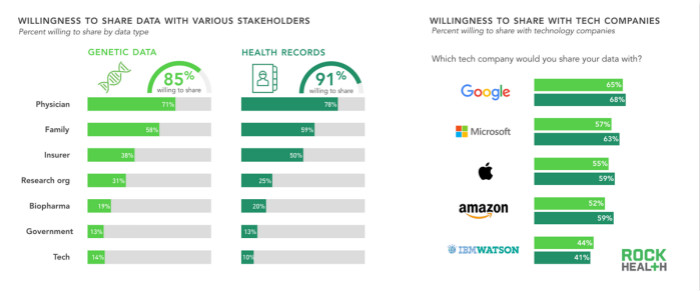
Users have a strong desire to share their medical records and genetic data with doctors and their families, which means that trust is very important. The willingness of users to provide medical data to insurance companies and the willingness to share them with family members are the same, but for genetic data, trust in insurance institutions is significantly reduced. This may be due to consumer concerns about the use of genetic data to block access to premiums. Although the 2008 Anti-Genetic Discrimination Act explicitly prohibits medical insurance organizations from using genetic data as a reason for refusing to insure or increase premiums, the Act does not include long-term insurance and life insurance. These national laws provide varying degrees of protection, but do not break consumer concerns.
In our survey, only 11% of respondents are willing to share their medical data with technology companies, which is lower than pharmaceutical companies and government agencies. Among the technology companies mentioned in the survey, Google is most trusted by consumers, although other companies, such as IBM and Apple, are better at genomics.
n= 1,060
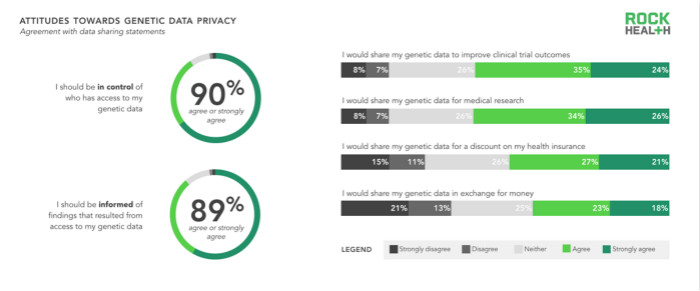
Nearly 90% of respondents believe they should have control over who can access their genetic data, and 89% of respondents want to be informed of the results of analyzing genetic data. But consumers are still concerned about sharing their genetic data, despite providing compensation such as lowering premiums, better clinical trial results, and cash rewards. In fact, sharing genetic data is negatively correlated with accepting cash rewards. However, as consumers become more aware of managing health and genetic data in the future, they may gradually accept shared genetic data, especially when there are compensation and reporting. Currently, the best way for genomics companies is to provide consumers with valuable examples to get more samples and data.
challenge
As mentioned above, consumers derive value from three types of genetic testing: clinical, health and lifestyle. Although there is great interest in genomics, many consumers do not have a clear value proposition about why genetic testing should be done. So how does the industry increase the effective use cases that can be used or increase the value to users? The industry is facing the problem of “chicken or egg firstâ€: more data is needed to get more valuable insights; more data is needed to get more consumers to buy.
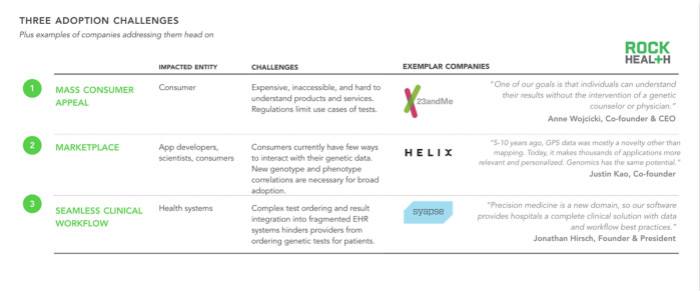
After talking to more than 30 companies, we found three ways to motivate consumers to buy behavior: First, genomics companies (such as 23andMe) should break down barriers to popular appeal through affordable, accessible products, encouraging those who Hesitant consumers buy. Similarly, companies should provide products that continue to create value for users after prolonged gene sequencing (such as providing undiscovered insights in the future) and even more effectively motivate consumers to undergo genetic testing – Helix has begun to do so. Goal efforts. The third method is the collection of information driven by doctors and medical systems. We have studied several innovative medical systems (including Intermountain Healthcare) and found that many healthcare organizations encourage a wider range of patient populations (not limited to specific patient populations, such as cancer patient populations) to use genetic testing. Intermountain is working closely with Syapse to launch products that streamline the clinical workflow of genetic testing.
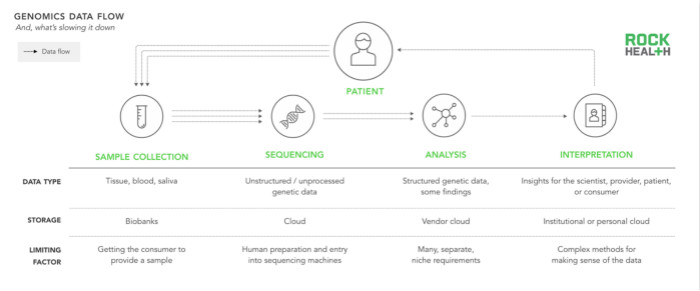
In addition to simply generating more data, the industry should improve all aspects of the entire genomics cycle by better absorbing, understanding, and reporting the value of the data. Historically, the development of the genomics cycle has been limited by the high cost of gene sequencing. But advances in life sciences technology have reduced the cost of sequencing, and the advancement of computing power and storage capacity (such as Amazon's Web Service) has also enabled most laboratories to meet computing and storage requirements.
According to our interviews, many scientists and engineers agree that the ability to interpret data or to identify genes and phenotypic data has become the key to limiting development speed. Although these problems can be solved by algorithms, this process requires highly specialized bioinformatics scientists to make minor adjustments to the test methods based on different assumptions. As Andrew Guo from Omicia said, "We are talking about a field where scientists spend hundreds of hours analyzing which variant is the most deadly and relevant."
So suppose the first two requirements: more high-quality data and data translation has been met, then other requirements?
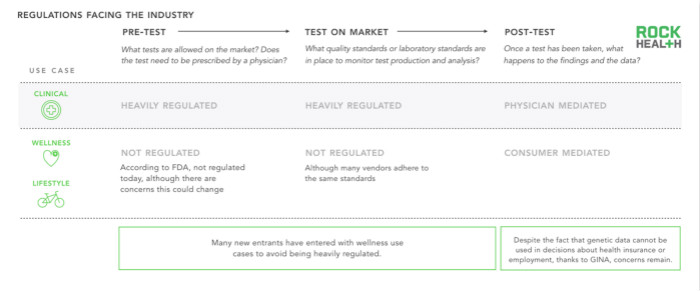
Two barriers to the application of genomics come from the lack of clear regulations and limited reimbursement programs. The corresponding regulations should be formulated in the early, middle and late stages of genetic testing.
Regulations dictate which test items are sold, how to apply genetic data, and these regulations need to be constantly changing, taking into account the fact that clinical use cases are regulated by the Food and Drug Administration. At the time of writing this report, if a claim is made for the diagnosis or treatment of a genetic test, the user cannot make the request and must be treated by a doctor. To date, the only bill that clearly defines the use of post-test information is the Anti-Genetic Discrimination Act (GINA), which states that genetic information cannot be applied to health insurance and recruitment discrimination.
In addition to the ever-changing laws, many institutions have also been affected, further complicating the process of genetic testing. The Centers for Medicare and Medicaid Services (CMS) stipulates that each accredited laboratory must have a Medical Laboratory Improvement Act Amendment (CLIA) certificate. The Food and Drug Administration (FDA) oversees genetic testing equipment to determine whether the test is accurate and reliable (analytical effectiveness), whether it has achieved medically meaningful results (clinical effectiveness), and whether the medical information it provides is correct. The patient has medical help (clinical utility). Some states also require genomics companies to develop and sell products within the state (such as New York State).
As genetic testing-related regulations continue to change, and health and lifestyle-related testing is relatively rare, a large number of start-up, consumer-oriented genomics companies have chosen to enter the market with non-clinical genetic products.
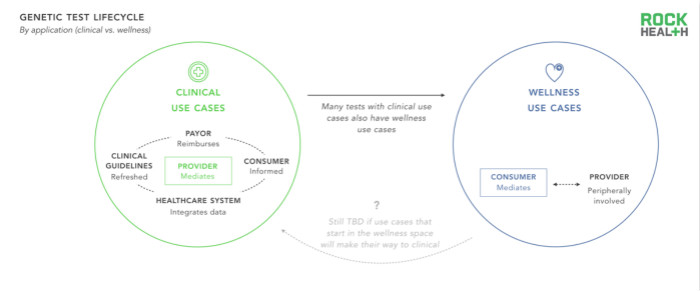
When genetic testing is in a closed-loop medical system (from doctor appointment testing, user reimbursement to entry of genetic data to electronic health records), the results of genetic testing can predict clinical decisions and influence the final medical outcome. Therefore, the effectiveness and cost-effectiveness of genetic testing has become possible, and it is expected to become the guiding principle for medical decision-making and medical reimbursement in the future. Many genomics companies choose to introduce health and lifestyle-related tests when they enter the market to avoid FDA review; however, these tests cannot be combined with a broader medical system. As a result, these genomics companies miss out on potential opportunities to get feedback loops, relying on traditionally more challenging channels—consumer payments.
As to whether genetic testing for health and lifestyle use cases can be applied in clinical treatment (the test itself still analyzes and reports the same gene, but the scope of its application will be expanded), will the relevant provisions of health testing be more stringent, or Whether health and lifestyle testing continues to be separated from clinical applications remains to be seen. Because medical systems still benefit from understanding which genetic testing programs can create medical value, they have a particularly accurate positioning to drive the needs of clinical testing.
Conclusion
Genomics companies that can solve censorship, expense reimbursement, and data acquisition problems can ultimately deliver tremendous value to patients and generate high returns. Consumers have always wanted and will be able to access their own genetic data. But whether doctors and experts continue to be middlemen in understanding genomics, or whether consumers can become genetic data stewards is not known. At present, it is clear that the medical system has a good positioning and has a profound impact on the development of genomics because the system can collect data and rely more and more on evidence to make clinical decisions. Genomics will continue to understand the three billion base pairs of the human genome, and we are happy to witness the significant impact of this science on the health care industry and people's health and well-being.
Source: Arterial Network
Insulin Syringes Needle,Disable Syringe,Monoject Syringe,10 Ml Syringe
FOSHAN PHARMA CO., LTD. , https://www.full-pharma.com